Key takeaways:
~ BH4 is an essential cofactor needed for the production of neurotransmitters, nitric oxide, and more.
~ Your BH4 levels are important for heart health, immune response, and cognitive disorders.
~ Genetic variants affect how likely you are to have low BH4 during times of oxidative stress or inflammation.
~ There are natural ways to improve low BH4 levels, as well as safety considerations.
Members will see their genotype report below, plus additional solutions in the Lifehacks section. Consider joining today.
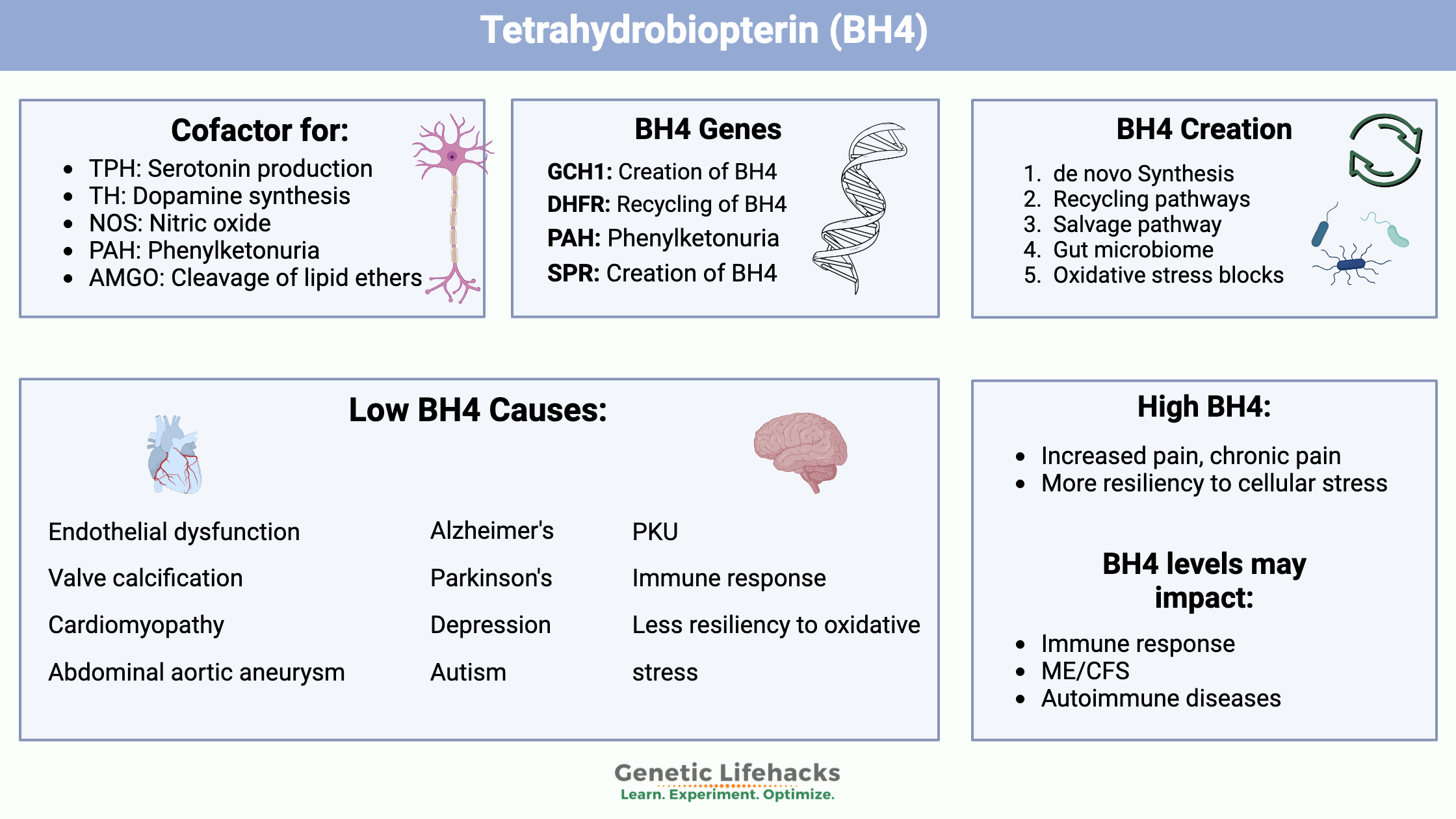
Tetrahydrobiopterin, BH4: Essential cofactor for neurotransmitters, NOS, and more
Tetrahydrobiopterin is an essential cofactor for enzymes involved in the biosynthesis of serotonin, dopamine, norepinephrine, epinephrine, melatonin, and nitric oxide.
The enzymes and pathways for which BH4 is an essential cofactor include:
- Tryptophan hydroxylase (TPH1 and TPH2 genes, serotonin production)
- Tyrosine hydroxylase (TH gene, essential for dopamine synthesis)
- Nitric oxide synthase (NOS3, makes nitric oxide)
- Phenylalanine hydroxylase (PAH, mutations cause phenylketonuria)
- Alkylglycerol monooxygenase (catalyzes the cleavage of lipid ethers into glycerol and the corresponding aldehyde)[ref]
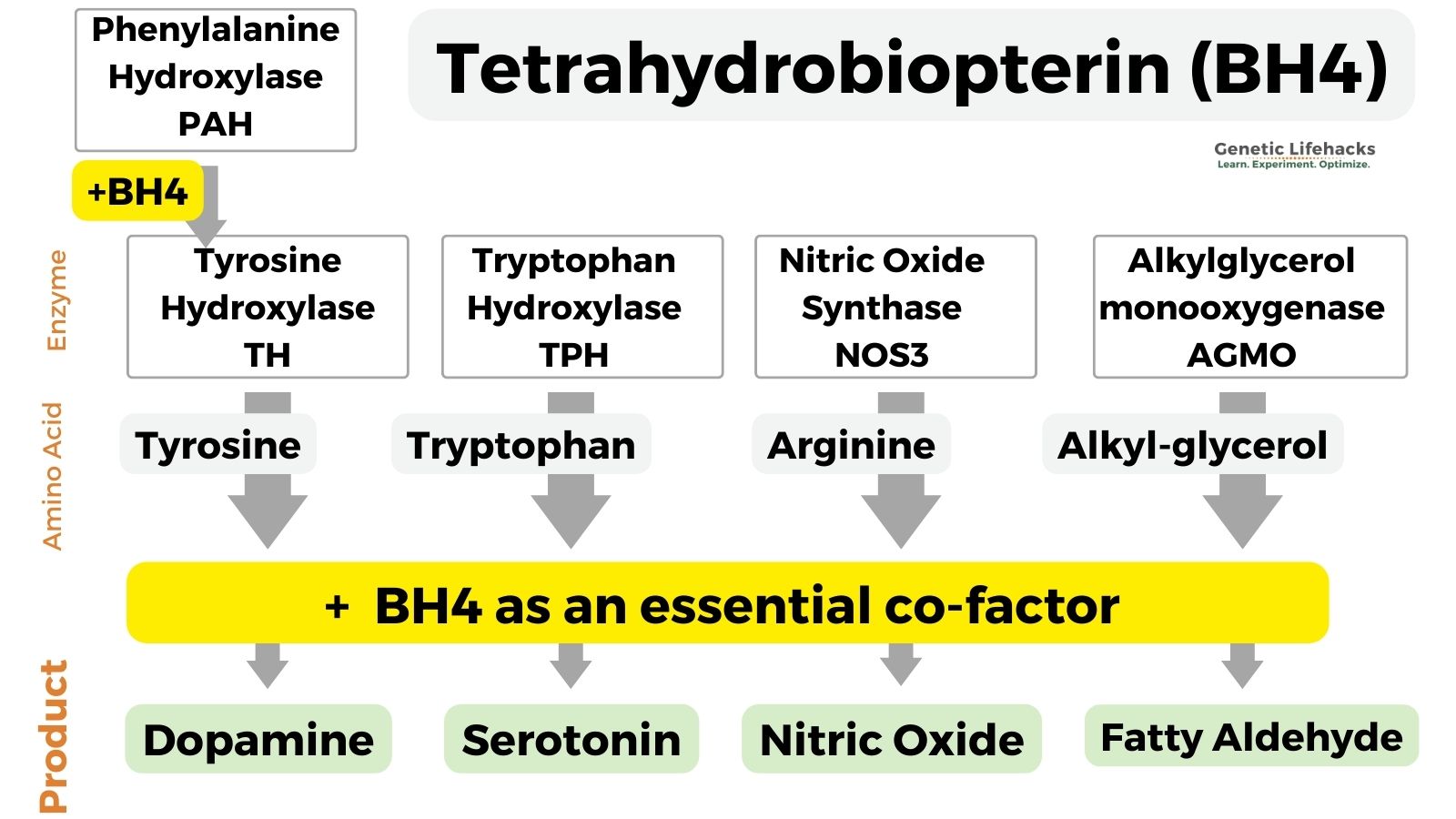
Here’s a graphical overview of the end products that depend on BH4:
This is a long article (bookmark it now so you can come back to it). Here’s where I’m going with it, so you can follow along:
I. What BH4 is used for in the body:
- BH4 as a cofactor in the creation of neurotransmitters
- Nitric oxide synthase, BH4, and vascular/cardiac function
- Phenylalanine and phenylketonuria
- AGMO and ether lipid metabolism
II. How BH4 is made, recycled, and oxidized:
- de novo Synthesis
- Recycling and salvage pathways
- Oxidative stress
- Gut microbiome
III. How BH4 interacts with the immune system
IV. Genotype report on the synthesis, recycling, and utilization of BH4
V. Lifehacks for increasing BH4 (and safety considerations)
1. Creation of neurotransmitters: BH4 in the synthesis of dopamine and serotonin
Let’s start by looking at the essential role of tetrahydrobiopterin (BH4) in neurotransmitter biosynthesis.
In the late 1960s, scientists discovered that BH4 is an essential cofactor in the way the body makes dopamine from tyrosine and serotonin from tryptophan.[ref]
Tyrosine is converted to dopamine by the enzyme tyrosine hydroxylase (TH gene) along with BH4 as a cofactor.
tyrosine + BH4 –> dopamine
Tryptophan is an essential amino acid that is converted to serotonin by the enzyme tryptophan hydroxylase (TPH1 and TPH2 genes).
tryptophan + BH4 –> serotonin
Tryptophan can also be converted to kynurenine, which can lead to quinolinic acid and neuroinflammation.
One theory is that low BH4 levels due to oxidative stress may shift tryptophan metabolism away from serotonin and toward the kynurenine pathway.[ref]
BH4 is also essential for the neurotransmitters norepinephrine and epinephrine since they are made from dopamine.
Similarly, BH4 is essential for melatonin, which is synthesized from serotonin.
Neurological Effects of BH4 deficiency
BH4 is an essential cofactor for the conversion of phenylalanine, and one (uncommon) cause of phenylketonuria is genetic BH4 deficiency. The inability to metabolize phenylalanine causes severe neurological effects on the developing brain.
Some studies point to low levels of BH4 in the cerebrospinal fluid of younger children with autism. In the 1990s, Japanese researchers found that in children 5 years old or younger with low BH4 levels, supplemental tetrahydrobiopterin significantly improved autism symptoms in some.[ref] (To be clear, not all children with autism have low BH4 levels, nor will all respond to BH4).
People with Alzheimer’s disease have been shown to have decreased levels of BH4 and its metabolites. In addition, neopterin (BH2), a derivative of BH4 produced during inflammatory conditions, is elevated. Mouse studies show that supplemental BH4 from middle age protects against the cognitive and metabolic deficits of Alzheimer’s disease.[ref]
Parkinson’s disease is caused by changes in the dopamine-responsive neurons. Genetic variants that affect BH4 production may affect dopamine production. Researchers have found that haplotypes, or combinations of variants, that decrease BH4 production are associated with earlier onset of symptoms in people with Parkinson’s disease.[ref]
Hypoxia is a lack of oxygen that can be caused by narrowed arteries, microclots, or anything that reduces blood or oxygen flow. In a cell study, BH4-deficient neurons were much more vulnerable to hypoxia-induced damage. In the BH4-deficient neurons, hypoxia reduced energy production by inhibiting mitochondrial complex IV activity.[ref]
In some people with depression, BH4 levels are low and BH2 levels are higher.[ref] In postmortem brain samples of people with persistent depression, BH4 levels were found to be low.[ref] A mouse model of BH4 deficiency showed that low BH4 can play a causal role in depression and anxiety. The researchers found decreased hippocampal serotonin and dopamine in the BH4-deficient mice, along with reduced nitric oxide formation.[ref] To be clear – not everyone with depression has low BH4. There are multiple root causes of depression.
BH4, inflammation, and neurological effects:
Under inflammatory conditions, such as a bacterial infection, the normal response is for BH4 to increase. It is also normal to have behavioral changes or sickness behavior when fighting off an infection.
A recent animal study showed that when mice are exposed to lipopolysaccharide (mimicking a bacterial infection), the need for BH4 increases. In animals exposed to lipopolysaccharide, there is less dopamine in the brain due to a reduced supply of BH4 in the striatum as BH4 is used elsewhere. This caused depressive-like symptoms in the animals. Supplemental BH4 restored the lost dopamine response in the brain.[ref]
In severe malaria, BH2 levels are increased and BH4 levels are decreased, indicating increased oxidative stress.[ref]
Chronic inflammation can cause depression in some people. BH4 comes into play here in several ways. First, increased inflammation could lead to lower BH4 levels, causing a decrease in serotonin production. Tryptophan, the precursor amino acid for serotonin, can also be converted to kynurenine. The kynurenine pathway can lead to an increase in quinolinic acid, which is a neurotoxin.[ref]
Related article: More on tryptophan, kynurenine, inflammation, and genetics
Higher BH4 and increased pain: trade-offs
Low levels of tetrahydrobiopterin have been the subject of much research, with many studies showing the benefits of increasing BH4 for cognitive and cardiac function.
However, higher levels of BH4 may have drawbacks. Trade-offs.
For example, in sensory neurons and nerve damage, higher BH4 levels lead to more pain.[ref]
Genetic variants associated with higher BH4 production are associated with increased pain in chronic pain conditions.[ref] Similarly, people with genetic variants that cause lower BH4 production are significantly less likely to have pain, such as chronic pain or neuropathy.[ref] (See your GCH1 variants in the genotype report section below.)
In addition, new research shows that BH4 may be involved in regulating miRNA that activates microglia in neuropathic pain.[ref][ref]
2. Nitric oxide synthase (NOS): BH4 as a cofactor to create nitric oxide (NO)
BH4 is an essential cofactor in the formation of nitric oxide from l-arginine. The reaction that produces endothelial nitric oxide involves the eNOS (nitric oxide synthase) enzyme plus BH4 as a cofactor.
Endothelial nitric oxide plays an important role in controlling the relaxation of blood vessels, which lowers blood pressure.
Researchers believe that BH4 acts both as a cofactor and has redox functions in the creation of nitric oxide.[ref]
Low BH4: When there isn’t enough BH4 to act as a cofactor for endothelial nitric oxide production, the eNOS activity shifts to producing superoxide, a strong pro-oxidant. This can cause oxidative stress and even trigger cell death.[ref]
Low levels of BH4 are associated with several types of cardiovascular disease, including high blood pressure, heart disease, and cardiac hypertrophy.
Here are some of the ways that low BH4 can affect the heart:
Low BH4 and aortic valve calcification:
In aortic valve calcification, the valve thickens and stiffens, causing it to leak with each heartbeat. This is somewhat common with aging, or it can be caused by infection in the endothelial cells of the heart.
Recent research shows that aortic valve calcification is caused by inflammatory factors and cellular regulation of osteogenesis.[ref] In other words, it’s not just a natural process of aging, but rather one that is driven by oxidative stress.
Research shows that “aortic valve BH4 concentrations and the BH4:BH2 ratio were significantly lower in calcific aortic valve disease patients than in controls.” The researchers found that in animals, BH4 supplementation can prevent heart valve calcification. In addition, folate, which acts to increase an enzyme in the salvage pathway for BH4 (more on this below), was also able to help attenuate the aortic valve calcification.[ref]
Cardiomyopathy due to tetrahydrobiopterin deficiency:
Deficiency of endothelial BH4 in the heart leads to NOS uncoupling and decreased endothelial production of nitric oxide. In an animal model, this caused mild cardiomyopathy. The lack of endothelial BH4 led to coronary blood vessel dysfunction and worsened outcomes for myocardial infarction.[ref]
Abdominal aortic aneurysm and BH4 deficiency:
Research in mice deficient in BH4 (due to GCH1 mutations) shows that tetrahydrobiopterin and eNOS are important in abdominal aortic aneurysms. In the BH4-deficient mice, researchers could induce the aneurysms (killing 79% of the mice) by giving them angiotensin II, which uncouples eNOS. Increasing DHFR, and thus BH4 recycling, prevented the formation of abdominal aortic aneurysms.[ref] However, in humans with DHFR variants, this recycling pathway may not work as well.[ref]
Systemic inflammation, BH4 response, and vascular function:
Systemic inflammation puts stress on the vascular system, leading to atherosclerosis in some people.
Endothelial nitric oxide is essential for vascular health and for resiliency during times of systemic inflammation. The body needs to be able to increase BH4 during times of inflammation so that there is enough available for eNOS responses to produce endothelial nitric oxide.
A unique study looked at how BH4 levels respond to systemic inflammation in people with genetic variants in a key gene for BH4 production. In people with variants (SNPs) that limited GHC1, their BH4 levels weren’t able to rise enough to respond to inflammation.[ref] (GCH1 gene in the genotype report section)
Interestingly, the researchers in the above study used vaccination as an acute model of systemic inflammation to determine how the GCH1 gene variant affected the increase (or lack thereof) of BH4 levels in the study participants.
Uncoupled endothelial NOS: vascular inflammation feedback loop
Normally, the production of endothelial NO is a good thing – it protects blood vessels and controls blood pressure. However, when oxidative stress is high in endothelial cells, nitric oxide can be rapidly inactivated by oxidation. This leads to more and increased oxidative stress, which is then exacerbated by endothelial NOS uncoupling, resulting in the production of superoxide. Uncoupling of eNOS is thought to be a key player in atherosclerosis.[ref][ref]
Tetrahydrobiopterin deficiency is a major cause of eNOS uncoupling.
The lack of BH4 may be caused by excessive oxidative stress, which causes BH4 to be oxidized to BH2 without sufficient recycling back to BH4.
As one research article puts it: “The overproduction of ROS (e.g. O2 ●¯ and subsequently peroxynitrite) by uncoupled eNOS, in turn, enhances the oxidation of BH4, creating a vicious circle”.[ref]
A 2021 study showed that BH2 levels, which indicate increased oxidative stress, increase with age. The study looked at BH2 and BH4 levels in healthy adults, and the results showed that people aged 60-70 had higher BH2 levels compared to younger adults. However, aging didn’t necessarily cause a decrease in BH4 levels.[ref]
iNOS: inducible nitric oxide synthase
While a lot of the research on tetrahydrobiopterin is focused on eNOS and heart disease, nitric oxide is also produced by the innate immune system to fight off pathogens.
Macrophages, a type of white blood cell, can be activated by interferon-gamma or lipopolysaccharide (from bacteria). When activated, macrophages can produce high levels of nitric oxide through inducible nitric oxide synthase. Similar to eNOS, iNOS also utilized BH4 as a cofactor.[ref]
3. Phenylalanine and PKU (Phenylketonuria):
Phenylalanine is an essential amino acid that is the precursor to tyrosine (used to make dopamine). The reaction that converts phenylalanine to tyrosine uses BH4 as an essential cofactor.
Phenylketonuria (PKU) is usually caused by mutations in the PAH gene, which codes for an enzyme that breaks down phenylalanine. In addition, mutations that affect the recycling and production of BH4 can cause high levels of phenylalanine. The accumulation of phenylalanine in infants and children with PAH mutations causes intellectual disability.[ref]
High levels of phenylalanine in the brain are neurotoxic. Researchers believe the cause is multifactorial due to inhibition of tyrosine and tryptophan hydroxylase, increased oxidative stress, altered lipid metabolism, and myelin sheath production.[ref]
BH4 (as sapropterin) is used as a drug to treat phenylketonuria. However, not everyone with phenylketonuria responds to BH4 treatment. It depends on which mutation is causing phenylketonuria. People with some residual PAH enzyme activity are more likely to respond to BH4.[ref][ref]
Related article: Phenylalanine and phenylketonuria mutations
4. Alkylglycerol monooxygenase AGMO gene: BH4-dependent reactions
Another enzyme for which BH4 is an essential cofactor is alkylglycerol monooxygenase. The gene encoding alkylglycerol monooxygenase (AGMO gene) was discovered in 2010; however, the enzyme has been known for decades
[ref]
Alkylglycerol monooxygenase is an enzyme that breaks apart certain types of lipids known as ether lipids.
In a broad sense, lipids are fats, but there are many different types of lipids that play different roles in the body. The category of lipids includes molecules that make up your cell membranes, fatty acids that are used for energy, and lipids that are signaling molecules (e.g. SPMs signal for the resolution of inflammation and prostaglandins that are pro-inflammatory).[ref]
Alkylglycerols are a type of ether lipid. They are part of the cell membrane, such as in neurons, and are also important in protecting the eye from cataracts. Ether lipids make up about 20% of the phospholipids in the body.[ref]
Alkylglycerol monooxygenase (AGMO gene) is the enzyme that breaks the ether bond in alkylglycerols.[ref]
Looking at the changes a gene mutation causes can give researchers a better understanding of what the gene does. In the AGMO gene, variants are linked to altered fasting glucose and fasting insulin levels. AGMO has also been implicated in neurodevelopmental changes and autism spectrum disorder. To date, 10 rare AGMO mutations have been linked to autism.[ref] Common variants (SNPs) in AGMO affect the immune response to specific pathogens, such as tuberculosis.[ref]
Immune response and AGMO:
Rare mutations in AGMO are also thought to cause changes in immune response and modulate levels of platelet activating factor (PAF).[ref]
PAF is a phospholipid mediator that causes platelet aggregation and dramatic airway inflammation (asthma-like symptoms). PAF is induced in response to bacteria, inflammation, and histamine activation of endothelial cells. [ref]
There is still a lot that researchers don’t know about AGMO, but they do know that AGMO is likely involved in macrophage activation (shift to inflammatory macrophage type). Additionally, animal and cell studies show that AGMO probably has a complex regulatory effect on PAF levels.[ref]
In the immune system, AGMO is expressed in macrophages. Researchers found that when they decreased AGMO expression in macrophages, it caused an increase in acylglycerol lipids, ceramides, and cardiolipins.[ref]
Leishmaniasis is a disease caused by a parasite transmitted by sandfly bites. The parasite replicates in macrophages in the liver, spleen, and bone marrow. A whole exome study of people who had relapsing leishmaniasis found that rare loss-of-function mutations in AGMO were likely the cause of the inability to clear the infection in the macrophages.[ref]
BH4 Deficiency Symptoms:
For people with rare genetic mutations causing severe BH4 deficiency, the symptoms can be significant and are usually present from birth. Since BH4 is a cofactor for phenylalanine metabolism, high phenylalanine is usually detected in newborn screenings. Untreated, BH4 deficiency with high phenylalanine can lead to developmental delays, muscle dysfunction, cognitive impairment, and sometimes epilepsy.[ref]
Rare mutations in different genes in the BH4 pathway can cause different genetic conditions. For example, some developed progressive encephalopathy while others may have spontaneous movement disorders.[ref]
Psychological and behavioral disturbances, such as ADHD, anxiety, depression, and aggression, are also common with BH4 deficiency, even if treated for high phenylalanine. Researchers assume this is due to the changes to neurotransmitter levels.[ref]
Beyond those rare and severe deficiency symptoms, a decrease in tetrahydrobiopterin also can play a role in the conditions listed above, such as altered nitric oxide creation, heart disease, immune system changes, and neurological conditions. Genetic variants that decrease BH4 levels are fairly common and found in more than 30% of the population.
Animal studies show that a 50% decrease in BH4 (due to GCH1 deficiency) causes primary gestational hypertension, but not preeclampsia (no kidney dysfunction).[ref]
Creation of BH4 in the body:
We’ve covered much of what BH4 is used for in the body, so now let’s dive into how it is synthesized.
Three things affect the availability of BH4:
- How much is made (de novo synthesis)
- How much is needed and used in reactions
- How much of it is recycled or regenerated (salvage route)
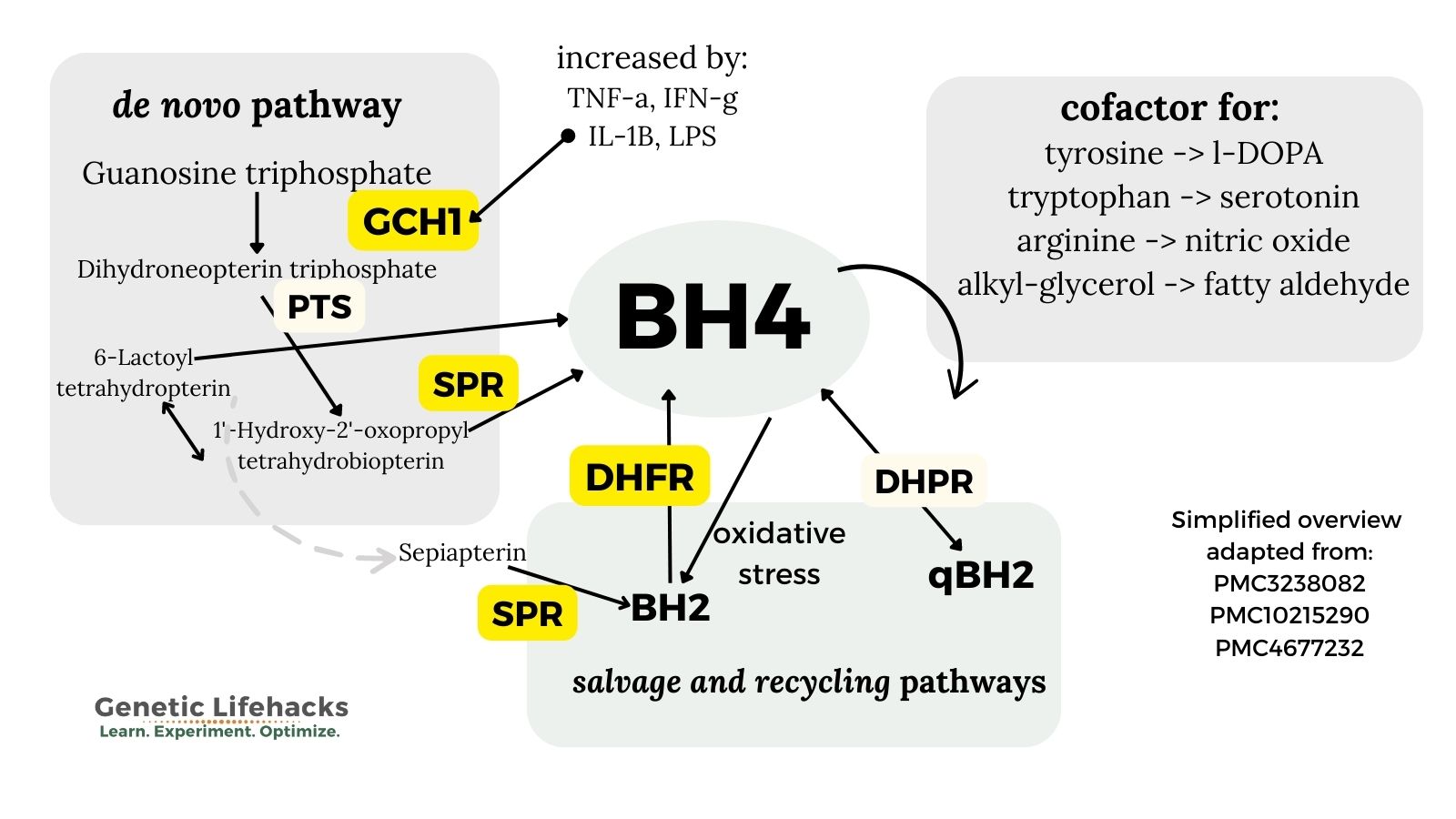
1. de novo synthesis of BH4:
BH4 is formed from GTP (guanosine-5′-triphosphate) in a three-step process. GTP is an abundant molecule used as a building block for other molecules, such as RNA.
The three steps in the synthesis of BH4 all use different enzymes that can be affected by genetic variants:
- The first step uses GTP cyclohydrolase I (GCH1 gene, in the genotype report)
- The second step uses 6-pyruvoyltetrahydrobiopterin synthase (PTS gene)
- The final step is catalyzed by sepiapterin reductase (SPR gene).[ref]
Variants in the GCH1 and SPR genes impact the availability of BH4.
The body controls BH4 levels through a feedback loop. GCH1 activity is negatively regulated by increased BH4. [ref]
2. Recycling and salvage route:
BH4 can also be regenerated from BH2, which is the oxidized form. [ref] The salvage and recycling pathways involve two enzymes: DHFR (also involved in the folate pathway) and DHPR (also known as QDPR).
The salvage pathway is important not only to produce more BH4, but also to reduce BH2 levels. Excess BH2 or an altered BH2:Bh4 ratio can lead to the generation of superoxide. Superoxide is a powerful oxidant that can cause oxidative stress in a cell.
The salvage pathway uses the DHFR (dihydrofolate reductase) enzyme to reduce (redox reaction) BH2 back to BH4. [ref]
The DHFR enzyme that converts BH2 back to BH4 is also used in the folate pathway. It is one of the final steps in the conversion of folic acid, a synthetic form of folate in fortified foods and vitamins, to the active form, methylfolate, which is used in the methylation cycle.
Methotrexate, a drug used to treat certain cancers and autoimmune diseases, inhibits the DHFR enzyme. Genetic variants in DHFR influence whether folic acid accumulates in the bloodstream in an unmetabolized form.
Research suggests that folic acid may benefit the heart and nitric oxide production by increasing DHFR enzyme production, thereby increasing the recycling of BH4.[ref][ref] However, some studies indicate that in people with DHFR variants (see the genotype report) excess folic acid intake may feed back and inhibit DHFR capabilities to increase BH4.[ref]
This same DHFR pathway is used for dietary folate, so increasing dietary folate may be another approach to increasing DHFR enzyme production.
Related article: DHFR, MTHFR, and folic acid (read about the pros and cons of folic acid)
3. Degradation of BH4 by oxidative stress:
During periods of high oxidative stress, BH4 is oxidized to BH2 at a higher rate.[ref]
Research shows that H2O2 (peroxide), interferon-gamma (IFNγ), tumor necrosis factor alpha (TNFα), and lipopolysaccharide (LPS, from bacteria) all increase the need for BH4.[ref]
What causes oxidative stress? This is a huge topic…
Causes of oxidative stress that have research directly linking them to increased nitric oxide or decreased BH4 include:
- BPA (a component of some plastics)[ref]
- Aluminum (also inhibits DHPR in BH4 recycling)[ref]
- Perfluorinated substances (PFAS, PFOAs)[ref]
- Vaccination (Salmonella typhi vaccine)[ref]
- Chemotherapy [ref]
- Interferon-alpha treatment[ref]
- Arthritis and rheumatic diseases[ref]
- Persistent viral infection, such as in some long Covid patients, hepatitis C[ref][ref]
- Smoking[ref]
An animal study that used a mouse model with genetically reduced GCH1 enzyme found that low BH4 also decreases Nrf2 activation. The Nrf2 pathway is part of phase II detoxification. It is activated by certain toxins as well as oxidative stress. [ref]
Related article: Nrf2 pathway genes
4. Gut microbiome production of BH4:
Researchers noticed that mice bred to be deficient in the GCH1 enzyme still had more tetrahydrobiopterin than they theoretically should. Since biopterin is not available in their diet, the researchers tested the mice’s gut microbes to see if they produced BH4. The results showed that both the mouse microbiome and human gut microbes do produce BH4. Bacteria in the phylum Actinobacteria, which includes Bifidobacterium longum and Bifidobacterium adolescentis, generated BH4. [ref]
Additionally, animal research shows that berberine promotes the production of BH4 through interaction with gut bacteria. Enterococcus faecalis and Enterococcus faecium bacteria along with berberine increased BH4 and increased dopamine in a mouse model of Parkinson’s.[ref]
Rabbit trail: To me, this raises questions on what the effect of an acute inflammation event along with a decrease in bifidobacteria has on people with GCH1 genetic variants that reduce BH4 production. I’m thinking about the study using a typhoid vaccine as a model of acute inflammation that showed people with GCH1 variants weren’t able to increase BH4 levels enough to prevent endothelial dysfunction.[ref] Add to this the reduction in Bifidobacterium seen in severe SARS-CoV-2 infection[ref]
The Immune System and Tetrahydrobiopterin:
I mentioned above in the Nitric Oxide section that BH4 can be oxidized to BH2 during inflammation and that AGMO plays a role in modulating platelet-activating factor in immune system activation. I’ll discuss the role of BH4 in the immune response in more detail here.
GCH1, which is normally the rate-limiting enzyme for BH4 synthesis, is increased up to 100-fold by interferon-gamma or IL-1B (usually in response to a pathogen). At this point, the other enzymes in the biosynthetic pathway become the rate-limiting factors[ref]
Interaction with the immune system and mast cells:
Mast cells are a part of the innate immune response. They can respond rapidly to allergens and pathogens by degranulating and releasing histamine, serotonin, cytokines, and more.
Researchers have found that overexpression of GCH1, which drives higher BH4 production, causes increased histamine and serotonin release from mast cell degranulation.[ref]
Related articles: Mast cell activation genes & Histamine intolerance genes
Long Covid and BH4 deficiency:
A hypothesis paper on long Covid notes that patients have symptoms that parallel other chronic inflammatory syndromes associated with low BH4. The author contends that people with a marginal BH4 deficiency due to genetic variants in the GCH1 gene (listed below in the genotype report) may end up with uncoupled NOS. The early immune response involves nitric oxide, and the marginal BH4 deficiency then leads to uncoupled NOS and a self-perpetuating cycle of superoxide production and increased oxidative stress. The reduction in BH4 availability would also affect AGMO, possibly allowing for an increase in platelet activating factor.[ref – open access]
This hypothesis has not been tested in any studies that I can find, but there have been a few genetics studies on Long Covid that did not identify GCH1 as a likely candidate.
ME/CFS (chronic fatigue syndrome) and high BH4:
A recent study of 32 patients with ME/CFS found that they had higher BH4 levels than healthy controls. The authors of the study imply in the discussion section that higher levels of BH4 may be causing the pathology of chronic fatigue syndrome, especially orthostatic intolerance, due to the possible increase in nitric oxide. [ref]
My thoughts (not an expert, take this with a grain of salt!):
- One possibility is that the slightly higher BH4 levels in the ME/CFS patients could indicate ongoing oxidative stress or a pathogen that is causing an upregulation of interferon-gamma. For example, elevated BH4 levels could be a natural response to elevated interferon-gamma in a reactivated Epstein-Barr infection.[ref][ref] Research shows that the Epstein-Barr virus may play a role in ME/CFS in a subset of patients. Other research suggests that immune system dysregulation may play a role in ME/CFS pathology.[ref]
- Another option could be that higher BH4 levels could be suppressing ferroptosis, which is altered in Epstein-Barr viral infections.
I would like to see GCH1 variants sequenced in ME/CFS patients with Epstein Barr.
Epstein-Barr infection and ferroptosis:
The Epstein-Barr virus (EBV) causes mononucleosis if you get it as a teenager or young adult. It is a virus that 95% of adults have had, and it sticks around as a latent virus.
Ferroptosis is a form of cell death that depends on iron (hence the ‘ferro’) along with lipid peroxides.
Cell death is something that happens consistently and at different rates in different tissue types. It is important in the prevention of cancer. In ferroptosis, intracellular glutathione is depleted, and iron oxidizes some of the lipids. This in turn generates a large amount of ROS, which triggers cell death.[ref]
BH4 is one regulator of ferroptosis. Higher levels of BH4, such as through supplementation or genetic modification, can suppress ferroptosis and rescue the cells from death.[ref] This isn’t always a good thing, though, in the case of cancer.
To tie this back to Epstein-Barr, the virus can cause Burkitt’s lymphoma by converting B cells into immortalized cell lines that continue to express the virus. Normally, ferroptosis would cause the cells to be destroyed, but EBV suppresses ferroptosis by increasing BH4.[ref]
Rabies infections:
Rabies virus is usually fatal in humans due to encephalitis and cerebral spasms. Approximately 59,000 people die from rabies each year, mostly in Africa and Asia. The virus travels from the site of infection (usually an animal bite) through peripheral nerves to the central nervous system. It can take a month or more for symptoms to appear.
Low levels of BH4 have been found in the brains of rabies patients. There is now a protocol for treating people with rabies, and BH4, along with several other medications, is a part of the protocol. [ref]
While most people will not contract rabies, the studies of BH4 in the viral infection of the central nervous system may shed some light on other infections.
Research on rabid animals shows that BH4 administration helps control the excessive innate immune response. High levels of IL-6 cause irreversible damage to neurons in the CNS. BH4 helped modulate IL-6 and interferon-gamma levels. Controlling the innate immune response prevented excessive damage to neurons and allowed time for the adaptive immune response to fight the viral infection.[ref]
Autoimmune diseases and BH4 levels:
T cells are a type of immune cell that are part of the adaptive immune response. They can be activated by pathogens or cancer and help protect the body from both. In autoimmune diseases, T cells can either help maintain tolerance to the self or cause tissue damage by attacking the body’s own cells.
The GCH1 gene, which is the first step in the synthesis of BH4, is expressed in T cells. When T cells are activated, GCH1 is induced and BH4 levels increase.
Researchers discovered that inhibiting GCH1 reduced the number of activated T cells. Using a number of different mouse models of autoimmunity and allergy, the researchers showed that inhibiting GCH1 attenuated T-cell proliferation. In addition, increasing GCH1 or increasing BH4 caused increased T-cell activation and proliferation (not good, in some autoimmune diseases).[ref]
In people with multiple sclerosis (MS), researchers have found that they have low BH4 levels and high neopterin levels, suggesting “excessive consumption of BH4 presumably in activated immune cells”. However, the blood tests showed that GCH1 was not upregulated indicating a dysregulation.
Using a mouse model of MS, the researchers found that increasing the BH4 levels caused an increase in T cells infiltrating the spinal cord (not good). In addition, there were lipid changes in ceramides that could contribute to the disruption of the blood-brain barrier. [ref]
Autoimmune diseases vary widely in the immune response, and MS may be an outlier in responding negatively to increasing BH4.
- In systemic sclerosis, which causes endothelial dysfunction and fibrosis in organs, researchers found that BH4 may have beneficial effects. The study involved 12 patients who received oral BH4 or a placebo. The oral BH4 increased markers which indicated improved endothelial function.[ref] Note that the study was only looking at short-term effects (5 hours) and not long-term results.
- Another study in systemic sclerosis found that BH4 administration improved endothelial function and helped with exercise-induced changes in blood flow.[ref]
- In an animal model of ulcerative colitis, oral BH4 treatment decreased symptoms. Moreover, inhibiting BH4 caused a worsening of colitis.[ref]
- A cell study of lupus patients showed that eNOS mRNA was increased compared to healthy controls, but they had reduced nitric oxide production. When the endothelial cells were treated with BH4, nitric oxide production returned to normal.[ref]
Let’s move on to looking at how YOUR genes could impact BH4 levels.
BH4 Genotype Report
Please note: The current 23andMe version (v5 raw data) does not cover the genes involved in the synthesis of BH4.
This genotype report is broken into 3 parts: genes involved in the synthesis of BH4, genes involved in recycling BH4, and genes that use BH4 as a cofactor.
1) Creation of tetrahydrobiopterin:
GCH1 gene: encodes the enzyme needed for the first step and rate-limiting enzyme in the biosynthesis of BH4.
Common GCH1 variants:
The following three variants have been identified in a study as reducing the function of the GCH1 enzyme. The combination of the three variants causes a statistically significant decrease, and two copies of all three SNPs cause an 80% decrease in enzyme function.[ref] Note that these variants are often inherited together. Around thirty percent of the population carries one copy.
Check your genetic data for rs3783641 (23andMe v4; AncestryDNA):
- T/T: typical
- A/T: somewhat reduced BH4 production, especially in combination with T allele of rs8007267 and G allele of rs10483639; reduced endothelial function in vascular disease
- A/A: reduced enzyme function, reduced BH4 production, less pain under certain conditions; 80% reduction in enzyme function when inherited with rs8007267 TT genotype and rs10483639 GG genotype[ref][ref] increased vascular superoxide and reduced vasorelaxation in vascular disease[ref] protective against post-herpetic neuralgia (ongoing shingles pain)[ref]; lower levels of chronic pain[ref]
Members: Your genotype for rs3783641 is —.
Check your genetic data for rs8007267 (23andMe v4; AncestryDNA):
- C/C: typical
- C/T: somewhat reduced BH4 production, especially when inherited with A allele of rs3783641 and G allele of rs10483639; reduced endothelial function in vascular disease
- T/T: reduced enzyme function, reduced BH4 production; 80% reduction in enzyme function when inherited with rs3783641 AA genotype and rs10483639 GG genotype[ref][ref]; increased vascular superoxide and reduced vasorelaxation in vascular disease[ref]; lower levels of chronic pain[ref]
Members: Your genotype for rs8007267 is —.
Check your genetic data for rs10483639 (23andMe v4; AncestryDNA):
- G/G: typical
- C/G: somewhat reduced BH4 production, especially when inherited along with A allele of rs3783641 and T allele of rs8007267; reduced endothelial function in vascular disease
- C/C: reduced enzyme function, reduced BH4 production; 80% reduction in enzyme function when inherited with rs3783641 AA genotype and rs8007267 TT genotype[ref][ref]; increased vascular superoxide and reduced vasorelaxation in vascular disease[ref]; lower levels of chronic pain[ref]
Members: Your genotype for rs10483639 is —.
These next two variants may also alter BH4 levels.
Check your genetic data for rs10137071 (AncestryDNA)
- C/C: typical levels
- C/T: somewhat lower plasma biopterin levels; linked to schizophrenia patients (common genotype)
- T/T: slightly lower plasma biopterin levels; linked to schizophrenia patients[ref][ref]
Members: Your genotype for rs10137071 is —.
Check your genetic data for rs17128077 (AncestryDNA):
- C/C: typical
- C/T: increased risk of cleft lip
- T/T: increased risk of cleft lip[ref]
Members: Your genotype for rs17128077 is —.
Rare mutations in GCH1:
Please keep in mind that AncestryDNA does not guarantee clinical accuracy in their test data. There is a possibility of inaccurate data. Talk with your doctor to see about a clinical grade test before pursuing any medical changes based on this information.
Check your genetic data for rs104894433 (AncestryDNA):
- G/G: typical
- A/G: loss of function GCH1 mutation, dystonia[ref]
Members: Your genotype for rs104894433 is —.
Check your genetic data for rs137852633 (AncestryDNA; 23andMe v4 i5000643)
- G/G: typical
- C/G: carrier of a rare mutation linked to dopa-responsive dystonia[ref]
Members: Your genotype for rs137852633 is — or for i5000643 is —.
Check your genetic data for rs104894445 (AncestryDNA; 23andMe v4 i5000644)
- C/C: typical
- C/T: carrier of a rare mutation linked to dopa-responsive dystonia[ref]
Members: Your genotype for rs104894445 is — or for i5000644 is —.
Check your genetic data for rs104894434 (AncestryDNA; 23andMe v4 i5000652)
- A/A: typical
- A/G: carrier of a rare mutation linked to dopa-responsive dystonia[ref]
Members: Your genotype for rs104894434 is — or for i5000652 is —.
Check your genetic data for rs104894438 (AncestryDNA; 23andMe v4 i5000654)
- C/C: typical
- C/T: carrier of a rare mutation linked to dopa-responsive dystonia[ref]
Members: Your genotype for rs104894438 is — or for i5000654 is —.
Check your genetic data for rs104894437 (AncestryDNA; 23andMe v4 i5000655)
- T/T: typical
- A/T: carrier of a rare mutation linked to dopa-responsive dystonia[ref]
Members: Your genotype for rs104894437 is — or for i5000655 is —.
SPR gene: encodes sepiapterin reductase, an enzyme needed in the synthesis of BH4; rare mutations cause more serious problems
Check your genetic data for rs1876487 (23andMe v4):
- A/A: earlier age of onset in Parkinson’s disease[ref]
- A/C: no impact on Parkinson’s age of onset
- C/C: typical
Members: Your genotype for rs1876487 is —.
Check your genetic data for rs121917747 (AncestryDNA, i5004360 23andMe v4)
- A/A: typical
- A/T: carrier of a rare SPR mutation[ref]
Members: Your genotype for rs121917747 is — or for i5004360 is —.
Check your genetic data for rs121917746 (AncestryDNA)
- C/C: typical
- C/T: carrier of a rare SPR mutation[ref]
Members: Your genotype for rs121917746 is —.
Check your genetic data for rs104893666 (AncestryDNA, i5004361 23andMe v4)
- C/C: typical
- C/T: carrier of a rare SPR mutation[ref]
Members: Your genotype for rs104893666 is — or for i5004361 is —.
2) Recycling of BH4:
DHFR gene: encodes dihydrofolate reductase, an enzyme that converts dihydrofolate (from folic acid or folate) into tetrahydrofolate. It also converts folic acid into dihydrofolate.
DHFR is also utilized in converting BH2 to BH4.
Check your genetic data for rs70991108 (23andMe v5 only):
- I/I (or AA): typical DHFR variant
- D/I: carrier of one copy of a deletion in part of the DHFR gene
- D/D: carrier of two copies of a deletion in part of the DHFR gene; more likely to have unmetabolized folic acid when consuming more than 500 mcg/day[ref]
Members: Your genotype for rs70991108 is —.
Check your genetic data for rs1650697 (23andMe v4, v5; AncestryDNA):
- A/A: decreased DHFR expression[ref];
- A/G: decreased DHFR expression
- G/G: a more common variant
Members: Your genotype for rs1650697 is —.
3) Enzymes that use BH4 as a Cofactor
AGMO gene: encodes an enzyme that utilizes BH4 as a cofactor in the metabolism of alkyl-glycerols
Check your genetic data for rs916943 (23andMe v4; AncestryDNA):
- C/C: typical
- C/T: increased susceptibility to tuberculosis[ref]
- T/T: increased susceptibility to tuberculosis[ref]
Members: Your genotype for rs916943 is —.
TPH2 gene: codes for the enzyme that converts tryptophan to 5-HTP, which then gets converted to serotonin in the brain. Read the full article on Tryptophan genes
Check your genetic data for rs4570625 (23andMe v4, v5; AncestryDNA):
- G/G: typical (most common genotype), less tryptophan conversion to serotonin, slightly higher risk of ADHD[ref] a higher risk of depression, suicidal depression[ref][ref]
- G/T: somewhat decreased risk of depression
- T/T: generally decreased risk of depression[ref], less aggressiveness and lower anxiety[ref] lower neuroticism[ref]
Members: Your genotype for rs4570625 is —.
Check your genetic data for rs11178997 (23andMe v4; AncestryDNA):
- T/T: typical
- A/T: somewhat increased risk of depression
- A/A: increased risk of depression and suicide[ref][ref]
Members: Your genotype for rs11178997 is —.
Check your genetic data for rs1843809 (23andMe v5; AncestryDNA):
- G/G: decreased risk of depression[ref]
- G/T: decreased risk of depression
- T/T: typical
Members: Your genotype for rs1843809 is —.
Check your genetic data for rs4290270 (23andMe v4; AncestryDNA):
- T/T: circadian disruption in people with depression[ref]
- A/T: typical
- A/A: higher risk of depression (Chinese study)[ref]
Members: Your genotype for rs4290270 is —.
NOS3 gene: encodes nitric oxide synthase; Read the full article on Nitric Oxide Synthase genes
Check your genetic data for rs891512 G24943A or IVS25+15 (23andMe v5; AncestryDNA):
- G/G: typical (more common allele)
- A/G: higher blood pressure, increased risk of coronary artery disease
- A/A: higher blood pressure[ref][ref], increased risk of coronary artery disease[ref]
Members: Your genotype for rs891512 is —.
Check your genetic data for rs1800779 (23andMe v4, v5; AncestryDNA):
- A/A: typical
- A/G: decreased NOS3 expression (compared to AA); increased risk of high blood pressure and cardiovascular disease
- G/G: decreased NOS3 expression (compared to AA); increased risk of high blood pressure and cardiovascular disease[ref], increased risk of primary open-angle glaucoma (women)[ref]
Members: Your genotype for rs1800779 is —.
Check your genetic data for rs4496877 (23andMe v4; AncestryDNA):
- G/G: typical
- G/T: increased risk of hypertension in older males (Chinese population)[ref], increased bilirubin in people who are older or who drink[ref]
- T/T: increased risk of hypertension in older males (Chinese population), increased bilirubin in people who are older or who drink[ref]
Members: Your genotype for rs4496877 is —.
Check your genetic data for rs2070744 (23andMe v5):
- C/C: increased risk of coronary artery disease (Asian, Caucasian)[ref], increased risk of osteoporosis[ref], decreased risk of hemorrhagic stroke, atrial fibrillation[ref][ref]
- C/T: increased risk of hypertension
- T/T: typical
Members: Your genotype for rs2070744 is —.
Lifehacks: Supplements and Diet for Tetrahydrobiopterin
The big picture in looking at BH4 shows that it is essential for neurotransmitter and nitric oxide production.
For cardiovascular health, it seems that increasing BH4 and/or decreasing endothelial oxidative stress will promote health. Oxidative stress decreases BH4 levels in the endothelium, driving the uncoupling of nitric oxide synthase and the production of more oxidative stress.
We need BH4 in the right amount, and a deficiency may be present in genetically susceptible people during times of immune activation. For some autoimmune diseases, it may be that the suppression of BH4 is a natural way that the body compensates for the overactivation of the immune response.[ref] In other immune dysregulation situations, it may be that increasing BH4 is helpful.
Safety of boosting BH4:
I want to first touch on safety concerns when upregulating the pathways that BH4 impacts.
First, be sure you know what you’re doing and talk with your doctor – especially if you have a mood disorder, medical condition, or are on prescription medication – before messing with BH4 since it is a rate-limiting factor in the production of neurotransmitters.
Also, be sure to talk with your doctor or pharmacist if you are on methotrexate, which blocks DHFR, the enzyme that recycles BH4.
Second, I don’t know if raising BH4 levels is a good idea for cancer. One research study that may be concerning shows that high GCH1 (limiting factor in the creation of BH4) is linked to reduced survival in women with triple-negative breast cancer.[ref] Not all cancers are the same, though. In mice, increasing BH4 boosts antitumor activity with T cells.[ref]
My final thought on increasing BH4 is that it may increase pain levels in someone with a chronic pain condition. Genetic variants that decrease tetrahydrobiopterin synthesis are associated with lower pain levels.
Clinical trial for ME/CFS and Long Covid:
If you are a long Covid or ME/CFS patient, you may want to check into this Stanford study on BH4 levels. (Currently recruiting, summer 2023)
Research on supplements for boosting tetrahydrobiopterin:
Related Articles and Topics: