Key takeaways:
~ Heat shock proteins (HSPs) are cellular chaperones activated by stressors like heat, cold, UV light, infections, and nutrient deprivation to protect and stabilize other proteins.
~ Repeated heat exposure, such as saunas or hot yoga, significantly boosts HSP levels, improving resilience, metabolism, and stress tolerance.
~ While HSPs are protective against neurodegeneration and aging, they can also promote viral replication and protect cancer cells.
~ Genetic variants can impact your natural heat shock protein production.
What are heat shock proteins?
Resilience. Survival. Flexibility. Our cells need to survive in all kinds of conditions – from cold to heat, nutrient deprivation to toxic insults. Heat shock proteins are at the heart of cellular resilience.
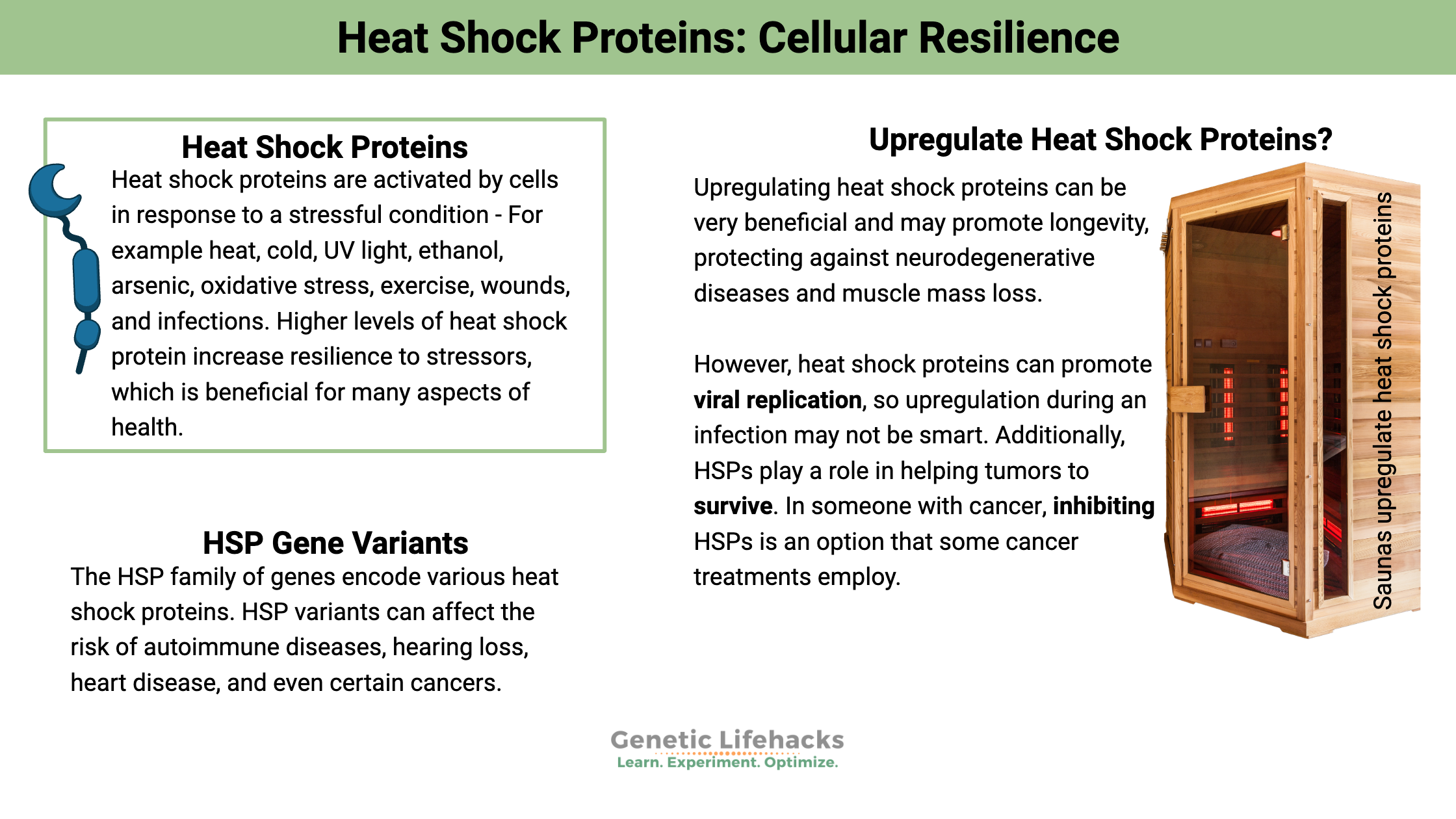
Heat shock proteins are activated by cells in response to a stressful condition, such as exposure to high heat. They were named ‘heat shock’ due to their discovery in the 1960s as a protein that is elevated under heat stress. However, it isn’t just heat that activates these proteins. Stressors such as cold, UV light, ethanol, arsenic, oxidative stress, exercise, wounds, infections, nutrient deprivation, and tissue remodeling also involve these proteins.[ref]
Heat shock proteins act as ‘chaperones’, a cell biology term meaning that they help stabilize and ensure the correct folding of other proteins under stress conditions. These resilience champions come alongside and help the other proteins needed in cellular function.
In humans, the family of heat shock proteins include Hsp27, Hsp40, Hsp60, Hsp70 (or Hsp72), and Hsp90. (The numbers stand for their molecular weight.)
We aren’t special here: heat shock proteins are actually found in all eukaryotes – from plants to animals to fungi. Heat shock proteins help to promote survival in all life forms (except bacteria and archaea).
” As a stress protein, heat shock protein 70 (HSP70) plays a pivotal role in protecting cells against apoptosis, oxidative damage and genetic damage. In humans, three genes encode members of the HSP70 class: HSPA1A, HSPA1B and HSPA1L.”[ref]
Both Hsp70 and Hsp90 are found abundantly in most of our cells.
For example, Hsp90 is found in most cell types and makes up about 1-2% of total cellular proteins in the body. When cells are put under stress, though, the amount of Hsp90 can rise to 4-6% of the cellular protein content.[ref]
The heat shock proteins can work together to help fold a ‘client’ protein. Protein folding is essential to the protein’s function, and misfolded proteins can have detrimental effects.
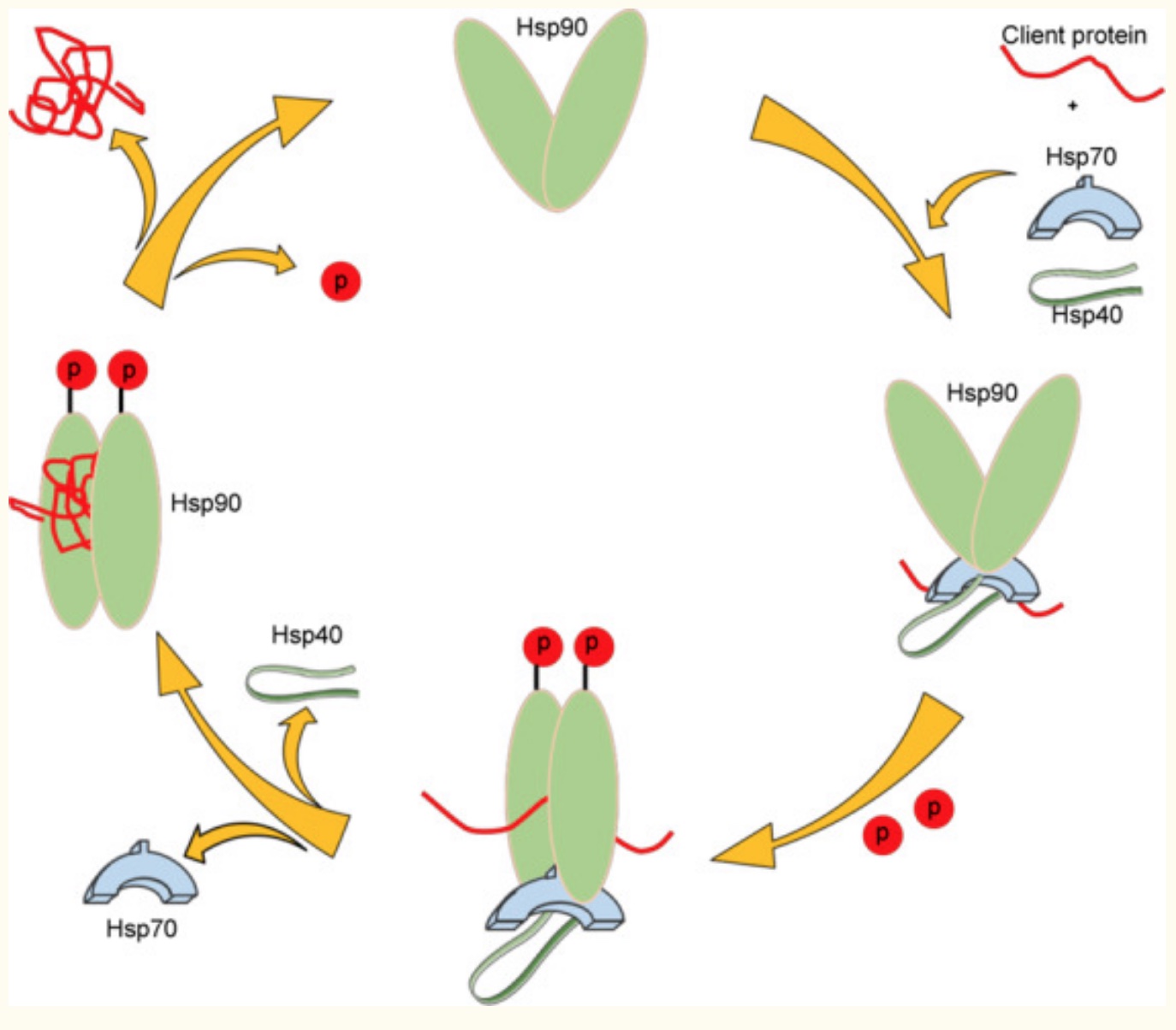
“To date, Hsp90 has been found to interact with over 200 client proteins, as well as ∼50 co-chaperones, making it a cornerstone in the cellular protein-folding machinery and an emerging target for the treatment of various disease states.”[ref]
What induces heat shock proteins?
I mentioned above that many different stressors could activate heat shock proteins, including heat.
Activators of heat shock proteins include:[ref][ref][ref]
- bacterial or viral infection
- oxidative stress
- elevated temperature
- DNA damage
- glyphosate
- arsenic
- long term cold exposure
- calorie restriction/starvation
Higher levels of heat shock protein increase resilience to stressors, which is beneficial for many aspects of health.[ref][ref] However, there are a couple of drawbacks to inducing heat shock proteins, in certain situations. We’ll get to that in a minute.
What temperature activates heat shock proteins?
Heat, of course, is an activator of heat shock proteins. Heat shock factor 1 (HSF1) regulates the heat shock response and activates the HSPs.
Animal studies showed that repeated exposure to mild heat stress (40°C or 104°F) for two hours a day for six days increased both Hsp70 and Hsp90 by around 40%. These increases caused beneficial mitochondrial adaptations.[ref]
Another study showed that exercising in the heat (42.5°C or 108°F) for 100 minutes for ten days in a row caused an increase in Hsp72. The study concluded that HPS elevations from day 6 through 10 showed that repeated heat exposure improves heat tolerance and reduces the risk of heat illness.[ref]
Research also shows that saunas increase heat shock proteins. For example, a study in healthy young adults, showed that just 30 minutes in a sauna increases HSP72 levels.[ref]
Heat exposure as a cure for depression:
Here’s one concrete example of a positive benefit of increasing HSPs:
Research points to neuroinflammation and/or mitochondrial dysfunction as one root cause of mood disorders.
Related article: Mitochondrial dysfunction and Depression
Upregulation of heat shock proteins may help decrease oxidative stress in neurons, acting in an anti-inflammatory manner. Repeated heat exposure, such as using a sauna several times a week, increases heat shock proteins.
A study that looked at the sauna habits of over 2,000 men found that sauna use 4+ times per week reduced mood disorder risk by almost 80%![ref]
Medications can also induce heat shock proteins. Valproic acid, a prescription medication used as a mood stabilizer and epilepsy drug, increases heat shock protein 70.[ref]
Neurodegenerative diseases and HSPs:
Protein misfolding is at the heart of several neurodegenerative diseases, including Alzheimer’s, Parkinson’s, and Huntington’s disease.
Heat shock protein 90 plays a key role in helping proteins fold correctly. HSP90 interacts as a chaperone in the brain to prevent the misfolding of many different proteins, including tau, huntingtin, and α-synuclein.[ref][ref] These are the proteins that are misfolded in Alzheimer’s (tau), Huntington’s (huntingtin), and Parkinson’s (α-synuclein) diseases.
On the other hand, the heat shock proteins may also help to stabilize and aggregate the already misfolded proteins in people who are already at a certain stage of neurodegeneration.[ref]
Whether increasing HSPs is beneficial in neurodegenerative diseases may depend on the stage of the disease. Researchers have found that Hsp90 helps clear out amyloid-beta plaque initially, but the chronic activation in the microglia leads to a pro-inflammatory cascade.[ref]
Double-edged sword: Cancer and Heat Shock Proteins
Heat shock protein 70 is great for protecting cells from toxins and stress. But when it comes to cancer, this can be a problem. Hsp70 (aka Hsp72) can interfere with chemotherapy, preventing it from working well. Within tumor cells, Hsp70 can work to protect cancerous cells from apoptosis and radiation therapy, which isn’t good.[ref]
Similarly, heat shock protein 90 inhibition is another target for reducing cancer cell growth. Hsp90 is essential for numerous proteins involved in malignant progression. Clinical trials are ongoing for Hsp90 inhibitors to facilitate cancer treatments.[ref][ref]
You may be wondering, as I was, whether increasing HSPs through sauna use could increase the risk of cancer. Two studies on this showed the opposite result:
One difference in the studies is that people who smoke are more likely to have cancerous or pre-cancerous cells in the body.
If you are undergoing treatment for cancer, talk with your oncologist before initiating any lifestyle changes aimed at increasing heat shock proteins.
HSPs and Viral Infections:
Viruses cannot replicate on their own. Instead, they use the host’s cellular replication pathways to reproduce the virus. One mechanism co-opted by some viruses is to use heat shock proteins, acting as chaperones, to help the viral proteins replicate.
In addition to its role in protein folding, Hsp70 is also important in viral infections. This heat shock protein helps to facilitate viral replication for certain viruses, including cytomegalovirus, rabies, RSV, HPV, and herpes simplex.[ref]
Enterovirus 71 causes hand, foot, and mouth disease. This viral infection upregulates Hsp27, and this heat shock protein facilitates viral replication. Interestingly, certain traditional Chinese medicine herbs block Hsp27 and may work as antivirals in this way.[ref]
HSF1: Controlling HSPs, increasing metabolism
The Heat Shock Factor 1 (HSF1) protein regulates the expression of heat shock proteins. When exposed to elevated temperatures (41°C or 106°F), the heat shock response activates HSF1. In turn, HSF1 increases the expression of heat shock proteins (HSP27, HSP40, HSP60, and HSP90). [ref]
HSF1, though, is more of a general way to increase stress response through activating genes involved in stress response, as well as DNA damage repair and detoxification. In addition to heat, HSP1 is activated by certain toxins and hypoxia (lack of oxygen).
Why am I rambling on about HSF1?
A new study published in Cell showed that increasing temperature in adipose (fat) tissue in mice causes metabolic changes and induced weight loss. The researchers think that the key is not to increase overall body temperature, which is a stressor to the heart and brain. Instead, heating just the adipose tissue induced HSF1 in the fat cells. This led to increased activation of beige adipose tissue, which is metabolically more active and resulted in weight loss.[ref]
The HSF1 gene is not included in 23andMe data, but variants in this gene are linked to neurodegenerative disorders and heat stress problems. The new study in Cell also found that an HSF1 variant that increased the function of the gene also improved metabolic function in people.
Heat Shock Proteins: Genotype Report
Lifehacks:
In certain situations, upregulating heat shock proteins can be very beneficial. HSPs may promote longevity, protecting against neurodegenerative diseases and muscle mass loss.[ref]
But… always keep in mind that heat shock proteins can promote viral replication, so upregulation during an infection may not be smart. Additionally, HSPs play a role in helping tumors to survive. In someone with cancer, inhibiting HSPs is an option that some cancer treatments employ.
5 ways to upregulating heat shock proteins without a sauna
Related Articles and Topics:
Intermittent Fasting: Benefits from changing Gene Expression
Nrf2 Pathway: Increasing the Body’s Ability to Get Rid of Toxins
References:
Amolins, M. W., and B. S. J. Blagg. “Natural Product Inhibitors of Hsp90: Potential Leads for Drug Discovery.” Mini Reviews in Medicinal Chemistry, vol. 9, no. 2, Feb. 2009, pp. 140–52.
—. “Natural Product Inhibitors of Hsp90: Potential Leads for Drug Discovery.” Mini Reviews in Medicinal Chemistry, vol. 9, no. 2, Feb. 2009, pp. 140–52.
—. “Natural Product Inhibitors of Hsp90: Potential Leads for Drug Discovery.” Mini Reviews in Medicinal Chemistry, vol. 9, no. 2, Feb. 2009, pp. 140–52.
—. “Natural Product Inhibitors of Hsp90: Potential Leads for Drug Discovery.” Mini Reviews in Medicinal Chemistry, vol. 9, no. 2, Feb. 2009, pp. 140–52.
—. “Natural Product Inhibitors of Hsp90: Potential Leads for Drug Discovery.” Mini Reviews in Medicinal Chemistry, vol. 9, no. 2, Feb. 2009, pp. 140–52.
—. “Natural Product Inhibitors of Hsp90: Potential Leads for Drug Discovery.” Mini Reviews in Medicinal Chemistry, vol. 9, no. 2, Feb. 2009, pp. 140–52.
Arawaka, Shigeki, et al. “Heat Shock Proteins as Suppressors of Accumulation of Toxic Prefibrillar Intermediates and Misfolded Proteins in Neurodegenerative Diseases.” Current Pharmaceutical Biotechnology, vol. 11, no. 2, Feb. 2010, pp. 158–66. PubMed, https://doi.org/10.2174/138920110790909713.
Bohush, Anastasiia, et al. “Hsp90 and Its Co-Chaperones in Neurodegenerative Diseases.” International Journal of Molecular Sciences, vol. 20, no. 20, Oct. 2019, p. 4976. PubMed Central, https://doi.org/10.3390/ijms20204976.
—. “Hsp90 and Its Co-Chaperones in Neurodegenerative Diseases.” International Journal of Molecular Sciences, vol. 20, no. 20, Oct. 2019, p. 4976. PubMed Central, https://doi.org/10.3390/ijms20204976.
—. “Hsp90 and Its Co-Chaperones in Neurodegenerative Diseases.” International Journal of Molecular Sciences, vol. 20, no. 20, Oct. 2019, p. 4976. PubMed Central, https://doi.org/10.3390/ijms20204976.
Boles, Richard G., et al. “Hurt, Tired and Queasy: Specific Variants in the ATPase Domain of the TRAP1 Mitochondrial Chaperone Are Associated with Common, Chronic ‘Functional’ Symptomatology Including Pain, Fatigue and Gastrointestinal Dysmotility.” Mitochondrion, vol. 23, July 2015, pp. 64–70. PubMed Central, https://doi.org/10.1016/j.mito.2015.05.002.
Bonvini, P., et al. “Consequences of Heat Shock Protein 72 (Hsp72) Expression and Activity on Stress-Induced Apoptosis in CD30+ NPM–ALK+ Anaplastic Large-Cell Lymphomas.” Leukemia, vol. 26, no. 6, June 2012, pp. 1375–82. www.nature.com, https://doi.org/10.1038/leu.2011.367.
Bourbeau, Kelsey Christian, et al. “Cardiovascular, Cellular, and Neural Adaptations to Hot Yoga versus Normal-Temperature Yoga.” International Journal of Yoga, vol. 14, no. 2, Aug. 2021, pp. 115–26. PubMed, https://doi.org/10.4103/ijoy.IJOY_134_20.
Dan, Xuelian, et al. “Hsp27 Responds to and Facilitates Enterovirus A71 Replication by Enhancing Viral Internal Ribosome Entry Site-Mediated Translation.” Journal of Virology, vol. 93, no. 9, May 2019, pp. e02322-18. PubMed, https://doi.org/10.1128/JVI.02322-18.
Hafen, Paul S., et al. “Repeated Exposure to Heat Stress Induces Mitochondrial Adaptation in Human Skeletal Muscle.” Journal of Applied Physiology, vol. 125, no. 5, Nov. 2018, pp. 1447–55. journals.physiology.org (Atypon), https://doi.org/10.1152/japplphysiol.00383.2018.
He, Meian, et al. “Functional SNPs in HSPA1A Gene Predict Risk of Coronary Heart Disease.” PloS One, vol. 4, no. 3, 2009, p. e4851. PubMed, https://doi.org/10.1371/journal.pone.0004851.
Hong, Yuhang, et al. “Antioxidative Status, Immunological Responses, and Heat Shock Protein Expression in Hepatopancreas of Chinese Mitten Crab, Eriocheir Sinensis under the Exposure of Glyphosate.” Fish & Shellfish Immunology, vol. 86, Mar. 2019, pp. 840–45. PubMed, https://doi.org/10.1016/j.fsi.2018.12.020.
Huang, Mei-Yu, et al. “Effect of Lavender Essential Oil on LPS-Stimulated Inflammation.” The American Journal of Chinese Medicine, vol. 40, no. 4, 2012, pp. 845–59. PubMed, https://doi.org/10.1142/S0192415X12500632.
Iguchi, Masaki, et al. “Heat Stress and Cardiovascular, Hormonal, and Heat Shock Proteins in Humans.” Journal of Athletic Training, vol. 47, no. 2, Apr. 2012, pp. 184–90. PubMed, https://doi.org/10.4085/1062-6050-47.2.184.
—. “Heat Stress and Cardiovascular, Hormonal, and Heat Shock Proteins in Humans.” Journal of Athletic Training, vol. 47, no. 2, Apr. 2012, pp. 184–90. PubMed, https://doi.org/10.4085/1062-6050-47.2.184.
Jacob, Pierre, et al. “The Heat-Shock Protein/Chaperone Network and Multiple Stress Resistance.” Plant Biotechnology Journal, vol. 15, no. 4, Apr. 2017, pp. 405–14. PubMed, https://doi.org/10.1111/pbi.12659.
—. “The Heat-Shock Protein/Chaperone Network and Multiple Stress Resistance.” Plant Biotechnology Journal, vol. 15, no. 4, Apr. 2017, pp. 405–14. PubMed, https://doi.org/10.1111/pbi.12659.
Jia, Yujie, et al. “Significance of Functional GRP78 Polymorphisms in Predicting the Onset of Type 2 Diabetic Peripheral Neuropathy in Chinese Population.” Neurological Research, vol. 37, no. 8, Aug. 2015, pp. 683–87. PubMed, https://doi.org/10.1179/1743132815Y.0000000054.
Kohan, Leila, et al. “HSPA1L and HSPA1B Gene Polymorphisms and Haplotypes Are Associated with Idiopathic Male Infertility in Iranian Population.” European Journal of Obstetrics, Gynecology, and Reproductive Biology, vol. 240, Sept. 2019, pp. 57–61. PubMed, https://doi.org/10.1016/j.ejogrb.2019.06.014.
Laukkanen, Jari A., et al. “Finnish Sauna Bathing Does Not Increase or Decrease the Risk of Cancer in Men: A Prospective Cohort Study.” European Journal of Cancer (Oxford, England: 1990), vol. 121, Nov. 2019, pp. 184–91. PubMed, https://doi.org/10.1016/j.ejca.2019.08.031.
Laukkanen, Tanjaniina, et al. “Sauna Bathing and Risk of Psychotic Disorders: A Prospective Cohort Study.” Medical Principles and Practice, vol. 27, no. 6, Mar. 2019, pp. 562–69. PubMed Central, https://doi.org/10.1159/000493392.
Li, Cun, et al. “Human Coronavirus Dependency on Host Heat Shock Protein 90 Reveals an Antiviral Target.” Emerging Microbes & Infections, vol. 9, no. 1, pp. 2663–72. PubMed Central, https://doi.org/10.1080/22221751.2020.1850183.
Li, Yanhong, et al. “Polymorphisms of Heat Shock Protein 70 Genes (HSPA1A, HSPA1B and HSPA1L) and Susceptibility of Noise-Induced Hearing Loss in a Chinese Population: A Case-Control Study.” PloS One, vol. 12, no. 2, 2017, p. e0171722. PubMed, https://doi.org/10.1371/journal.pone.0171722.
Marinova, Zoya, et al. “Valproic Acid Induces Functional Heat Shock Protein 70 via Class I Histone Deacetylase Inhibition in Cortical Neurons: A Potential Role of Sp1 Acetylation.” Journal of Neurochemistry, vol. 111, no. 4, Nov. 2009, pp. 976–87. PubMed Central, https://doi.org/10.1111/j.1471-4159.2009.06385.x.
Matz, J. M., et al. “Thermoregulatory and Heat-Shock Protein Response Deficits in Cold-Exposed Diabetic Mice.” The American Journal of Physiology, vol. 270, no. 3 Pt 2, Mar. 1996, pp. R525-532. PubMed, https://doi.org/10.1152/ajpregu.1996.270.3.R525.
Prodromou, Chrisostomos. “Mechanisms of Hsp90 Regulation.” Biochemical Journal, vol. 473, no. 16, Aug. 2016, pp. 2439–52. PubMed Central, https://doi.org/10.1042/BCJ20160005.
Radons, Jürgen. “The Human HSP70 Family of Chaperones: Where Do We Stand?” Cell Stress & Chaperones, vol. 21, no. 3, May 2016, pp. 379–404. PubMed Central, https://doi.org/10.1007/s12192-016-0676-6.
Singh, Ripudaman, et al. “Reduced Heat Shock Response in Human Mononuclear Cells during Aging and Its Association with Polymorphisms in HSP70 Genes.” Cell Stress & Chaperones, vol. 11, no. 3, Aug. 2006, pp. 208–15. PubMed Central, https://doi.org/10.1379/CSC-184R.1.
Tenkanen, L., et al. “Sauna, Dust and Migration as Risk Factors in Lung Cancer among Smoking and Non-Smoking Males in Finland.” International Journal of Cancer, vol. 35, no. 5, May 1985, pp. 637–42. PubMed, https://doi.org/10.1002/ijc.2910350511.
Thakur, Savant S., et al. “Therapeutic Potential of Heat Shock Protein Induction for Muscular Dystrophy and Other Muscle Wasting Conditions.” Philosophical Transactions of the Royal Society B: Biological Sciences, vol. 373, no. 1738, Jan. 2018, p. 20160528. PubMed Central, https://doi.org/10.1098/rstb.2016.0528.
Wan, Qianya, et al. “Stress Proteins: The Biological Functions in Virus Infection, Present and Challenges for Target-Based Antiviral Drug Development.” Signal Transduction and Targeted Therapy, vol. 5, July 2020, p. 125. PubMed Central, https://doi.org/10.1038/s41392-020-00233-4.
—. “Stress Proteins: The Biological Functions in Virus Infection, Present and Challenges for Target-Based Antiviral Drug Development.” Signal Transduction and Targeted Therapy, vol. 5, July 2020, p. 125. PubMed Central, https://doi.org/10.1038/s41392-020-00233-4.
Winder, T., et al. “GRP78 Promoter Polymorphism Rs391957 as Potential Predictor for Clinical Outcome in Gastric and Colorectal Cancer Patients.” Annals of Oncology: Official Journal of the European Society for Medical Oncology, vol. 22, no. 11, Nov. 2011, pp. 2431–39. PubMed, https://doi.org/10.1093/annonc/mdq771.
Wyler, Emanuel, et al. “Transcriptomic Profiling of SARS-CoV-2 Infected Human Cell Lines Identifies HSP90 as Target for COVID-19 Therapy.” IScience, vol. 24, no. 3, Feb. 2021, p. 102151. PubMed Central, https://doi.org/10.1016/j.isci.2021.102151.
Xie, Qiao-Mei, et al. “Hsp70 Gene Polymorphisms Are Associated With Disease Susceptibility and HRQOL Improvement in Chinese Han Population With Systemic Lupus Erythematosus.” Journal of Clinical Rheumatology: Practical Reports on Rheumatic & Musculoskeletal Diseases, vol. 26, no. 4, June 2020, pp. 134–41. PubMed, https://doi.org/10.1097/RHU.0000000000000986.
Yamada, Paulette M., et al. “Effect of Heat Acclimation on Heat Shock Protein 72 and Interleukin-10 in Humans.” Journal of Applied Physiology, vol. 103, no. 4, Oct. 2007, pp. 1196–204. journals.physiology.org (Atypon), https://doi.org/10.1152/japplphysiol.00242.2007.
Zhang, Jiao, et al. “Polymorphisms of Glucose-Regulated Protein 78 and Clinical Relevance of Neuroblastoma: Risk and Prognosis.” Journal of Cancer Research and Therapeutics, vol. 12, no. 3, Sept. 2016, pp. 1178–83. PubMed, https://doi.org/10.4103/0973-1482.193119.
Zhang, Ying, et al. “HSF1-Dependent Upregulation of Hsp70 by Sulfhydryl-Reactive Inducers of the KEAP1/NRF2/ARE Pathway.” Chemistry & Biology, vol. 18, no. 11, Nov. 2011, pp. 1355–61. PubMed Central, https://doi.org/10.1016/j.chembiol.2011.09.008.
Zheng, Hua-Chuan, et al. “The Meta and Bioinformatics Analysis of GRP78 Expression in Gastric Cancer.” Oncotarget, vol. 8, no. 42, Aug. 2017, pp. 73017–28. PubMed Central, https://doi.org/10.18632/oncotarget.20318.
Zong, Jinbao, et al. “HSPA1L Rs1061581 Polymorphism Is Associated with the Risk of Preeclampsia in Han Chinese Women.” Bioscience Reports, vol. 40, no. 2, Feb. 2020, p. BSR20194307. PubMed Central, https://doi.org/10.1042/BSR20194307.
Zubair, Mohammad, and Jamal Ahmad. “Heat Shock Protein 70 Gene Single Nucleotide Polymorphism and Diabetic Foot Ulcer. Is There Any Relationship?” Journal of Clinical Medicine, vol. 7, no. 8, July 2018, p. E187. PubMed, https://doi.org/10.3390/jcm7080187.
Zuehlke, Abbey D., et al. “Heat Shock Protein 90: Its Inhibition and Function.” Philosophical Transactions of the Royal Society B: Biological Sciences, vol. 373, no. 1738, Jan. 2018, p. 20160527. PubMed Central, https://doi.org/10.1098/rstb.2016.0527.