Key takeaways:
~ Depression can have multiple root causes, and one of those causes is mitochondrial dysfunction.
~ When mitochondria aren’t functioning optimally, they can create a lot of oxidative stress leading to overall elevated inflammatory cytokine production.
~ A systemic inflammation can then cause depression in some people.
~ Genetic variants related to mitochondrial dysfunction and response to stress can show you if you ar more susceptible to depression due to this root cause.
In this article, I will explore the research on how mitochondrial dysfunction relates to major depressive disorder. Included will be information on the causes of mitochondrial dysfunction as well as genetic variants that link the risk of depression to the mitochondria.
Members will see their genotype report below and the solutions in the Lifehacks section. Consider joining today.Mitochondria, oxidative stress, and depression risk:
Depression is a huge, global problem. Estimations show that depression has a lifetime prevalence rate of 20% worldwide. More than 1 million people per year die of suicide.[ref] These are startling statistics for a disease that doesn’t seem to have enough real solutions. SSRIs work for some people (about 20% more effective than placebo), and these medications reduce the risk of suicide by about 40% in adults (increases risk in teens).[ref][ref]
Obviously, we need better solutions that get to the root of the problem of depressive disorders. For some people, the answers may lie in the tiny powerhouse of the cell: the mitochondria.
Mitochondria are the ‘powerhouse of the cell’, constantly producing ATP. Your cells (except red blood cells) all have hundreds to thousands of mitochondria inside them, cranking out ATP for energy.
In this article, I’ll explain how and why mitochondrial function may be the root cause of depression for some people.
Key points:
- Stressed-out mitochondria produce a lot of ROS (reactive oxygen species), which causes oxidative stress
- Major depressive disorder is linked to inflammation, mitochondrial dysfunction, and oxidative stress.
- Mitochondrial dysfunction could cause changes to the way that the brain is wired.
Inflammation and depression:
What is known: Studies on depressed patients show increased inflammatory cytokines in many patients.[ref][ref][ref]
- For patients with major depressive disorder and high inflammatory cytokines, treating depression decreases cytokine levels.
- The corollary is also true: treating inflammation helps to decrease depression symptoms for many people.
Previously, I wrote about inflammatory cytokine genetic variants linked to depression and anxiety.
This article will go upstream of that process, looking at how oxidative stress – specifically oxidative stress from mitochondrial dysfunction – changes the brain in ways that can cause depression.
Depression as a complex, systemic disease:
Before we dive into the details of mitochondrial dysfunction in depression, I’d like to point out that for many people, depression goes hand-in-hand with other chronic diseases, pointing to depression as being a systemic problem in the body. Statistically, depression is linked with the following chronic diseases:[ref]
- cardiovascular disease
- cerebrovascular disease
- high blood pressure
- type 2 diabetes,
- autoimmune diseases
- Immune system impairment
- cancer
In other words, depression is not ‘all in your head’. At least not in the way that the pejorative term implies. You’ll notice that most of the chronic diseases linked to depression also involve mitochondrial dysfunction.[ref][ref][ref]
Research on depression focused for several decades on neurotransmitter levels, with a focus on serotonin. But serotonin has turned out not to be the problem or solution for many with depression.
A systemic point of view shows that changes in depression can physically alter other tissues — as well as change the brain.
Recent research shows that for people with long-term psychiatric illnesses, such as major depressive disorder, bipolar disorder, or schizophrenia, there are structural changes that take place in the brain.[ref] For me, the mental image is that neurotransmitters are acting like software, and altering the software code can alter mood. But the structural changes are more like changes to the hardware of the system — like switching from a Mac to a Windows PC. Changing the software (neurotransmitters) can alter the user experience (symptoms), but you are still limited by the hardware change. (I could further the metaphor and liken the Windows blue screen of death to some kind of psychotic break, but that may be taking it a bit too far.)
What is causing the structural changes – to the brain, to the heart, to the immune system?
Let’s look at the changes going on inside the cell, and then we will zoom out to look at the cause of those changes.
Mitochondrial dysfunction and ROS
Reactive oxygen species (ROS) are highly reactive molecules with a free oxygen that easily react with other molecules. Think about what happens if you leave something made of iron out in the air – it will react with oxygen to form rust pretty quickly. Reactive oxygen species will react automatically with other molecules, changing the other molecules.
Like most things in the body, you want just the right amount: too much ROS is bad, but some ROS is necessary for cell signaling.
Oxidative stress is the term for too much ROS. When ROS increases in a cell to the point of oxidative stress, damage can happen within the cell. This damage can take the form of oxidized lipids, protein oxidation, or damage to DNA which causes cell death.[ref]
In addition to being essential for energy production, mitochondria produce ROS and also play an important role in maintaining the right levels of ROS.
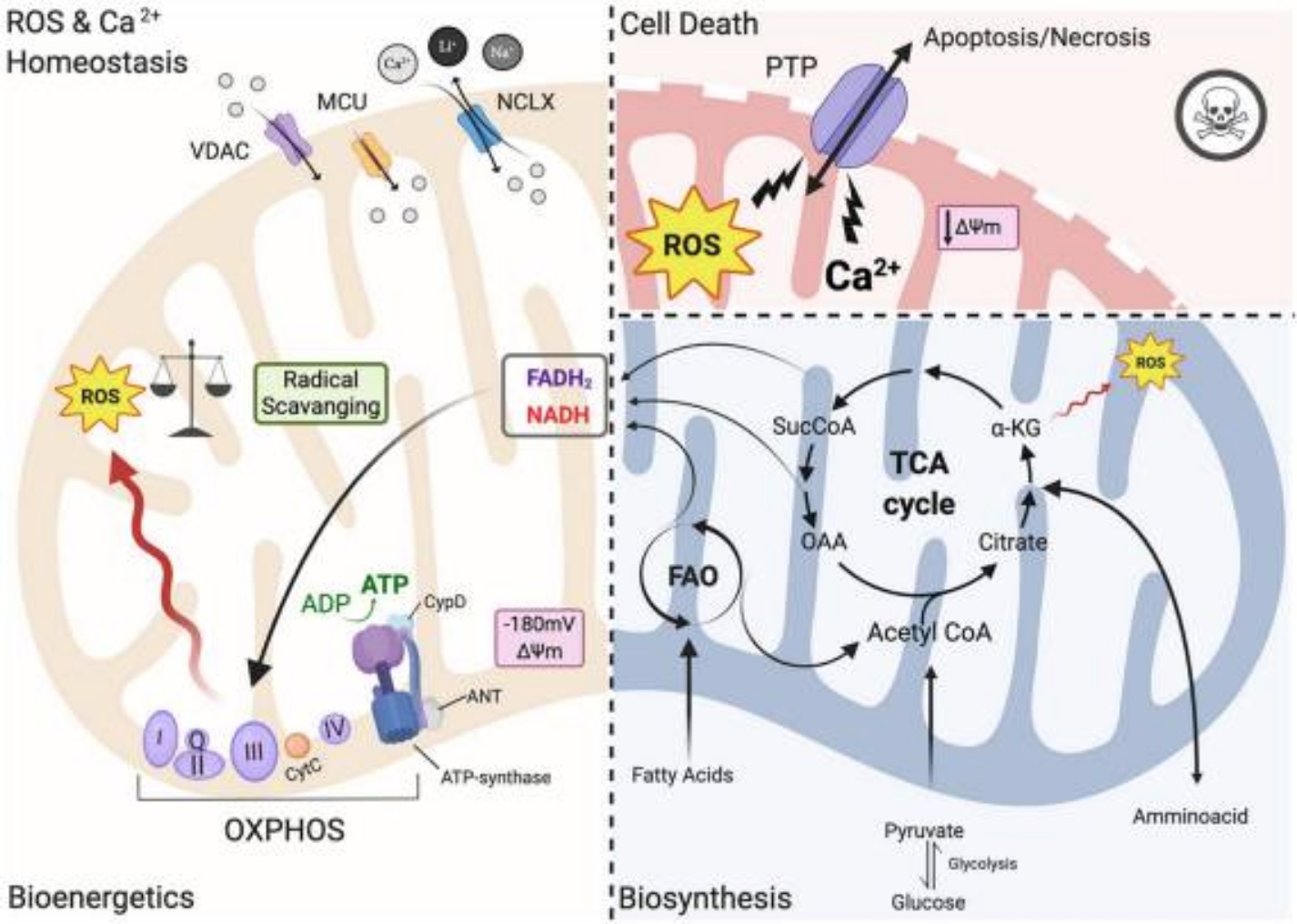
I mentioned before that mitochondria produce energy for the cell…One way that energy (as ATP) production occurs is through the electron transport chain. A series of reactions take place and move electrons across the inner membrane of the mitochondria. As a result of this process, ROS forms, and the cell then reduces the ROS into a non-reactive molecule. Here is a good video explanation of ROS production in normal mitochondrial function, where ROS is generated and then reduced by glutathione.
All is good when the ROS produced in the mitochondria is converted appropriately into a non-reactive molecule, and ROS levels are kept in check.
But there are several ways this process can go awry, increasing ROS to an inappropriate level. For example:
Chronic mild stress inhibits mitochondrial function at respiratory chain complexes I, III, and IV.[ref]
Let’s dig into chronic stress further:
Chronic stress: a cause of mitochondrial dysfunction.
Major depressive disorder is linked to ‘negative life events’. This scientific term covers the bad things that happen, such as the death of a loved one, divorce, major changes in relationships, job loss, financial problems, workplace stress, childhood abuse, chronic illness, injury, moving, and transitioning into adulthood.[ref]
Reduced sleep quality and quantity have strong links to major depressive disorder. Quality sleep is vital for proper cellular function, and lack of sleep on a regular basis is a chronic mild stressor.[ref]
Related article: Circadian rhythm and mood disorders
The body is made to handle short-term stress well. A series of biological actions take place when you have to escape from danger (Oh, no! A tiger!), and then you return to your normal baseline physiology. But chronic, low-level stress – whether mental or physical – causes physical changes.
Chronic stress can alter how the HPA axis regulates many body systems. The HPA axis includes the Hypothalamus (brain region), Pituitary (a gland in the brain), and Adrenals (produces adrenalin and cortisol). This feedback system controls your body’s reaction to stress and keeps everything hunky-dory when there is no stress.
Dysregulation of the HPA axis increases the production of ROS. A study of women who were under chronic stress showed oxidative damage when exposed to more stress – or in anticipation of more stress. Intriguingly, though, the women in the study who normally had low stress levels had the opposite physical reaction to a moderate stressor: their oxidative damage levels went down.[ref]
Animal studies allow researchers to see exactly how changing a single variable impacts biological systems. The research specifically shows that stressing out rats with mild stressors for 40 days causes increases in oxidative stress, including increases in SOD (superoxide dismutase).[ref] (We will return to SOD in a minute.)
How does the dysregulation of the HPA axis and altered cortisol levels cause mitochondrial dysfunction? Mitochondrial function, in part, is regulated by cortisol. Mitochondria have glucocorticoid receptors for detecting cortisol.
Cortisol affects neuronal mitochondrial function in a biphasic manner:
- Low, normal levels of cortisol increase mitochondrial function
- Chronically high levels of cortisol decrease mitochondrial function.[ref]
Chronic stress alters cortisol levels and thus can affect mitochondrial function in the brain.
Researchers now know that stress changes the way the brain functions, rewiring it. Plasticity in the brain refers to the ability to change and adapt as needed. The inability to return to a normal state when the stressor is removed indicates a failure of resilience.[ref]
Related article: HPA axis, cortisol, and stress
Is elevated cortisol the only way to cause mitochondrial dysfunction? Nope…
Other causes of oxidative stress:
Leaky gut:
The gut-brain axis refers to the link between what is going on in the intestines and brain changes (mood, brain fog, etc.) ‘Leaky gut’ causes an increase in bacterial pathogens moving across the gut barrier. This increases inflammatory cytokines and is linked to depression.[ref]
Iron build-up:
An excess of iron can cause an increase in oxidative stress.[ref] Research shows that iron accumulation in the brain can cause neuropsychiatric disorders.[ref]
Genetic connection to high iron: The body tightly regulates iron levels and usually only absorbs the right amount from foods. However genetic variants in the HFE gene can increase iron absorption.
Related article: Check your HFE gene
Alcohol use disorder:
Excessive alcohol consumption impacts the mitochondria. When consuming normal, low amounts of alcohol, your cells (mainly in the liver) use the alcohol dehydrogenase enzymes to break it down and eliminate it. But when drinking a lot of alcohol, the body will need the CYP2E1 enzymes. CYP2E1 enzymes work in the mitochondria and endoplasmic reticulum in response to high ethanol levels, and this produces a lot of ROS and oxidative stress.[ref][ref]
Related article: CYP2E1 gene variants | Alcohol use disorder
Calcium signaling and mitochondria:
In addition to their vital role in cellular energy production, mitochondria also participate in signaling between cells using calcium ions. In the brain, NMDA receptors and calcium signaling are responsible for long-term potentiation, which is the increase in synaptic strength in the brain upon stimulation. Essential, long-term potentiation is how we learn and remember.[ref] Mitochondrial function is essential for long-term potentiation, both through ATP production and via increasing calcium ions.[ref]
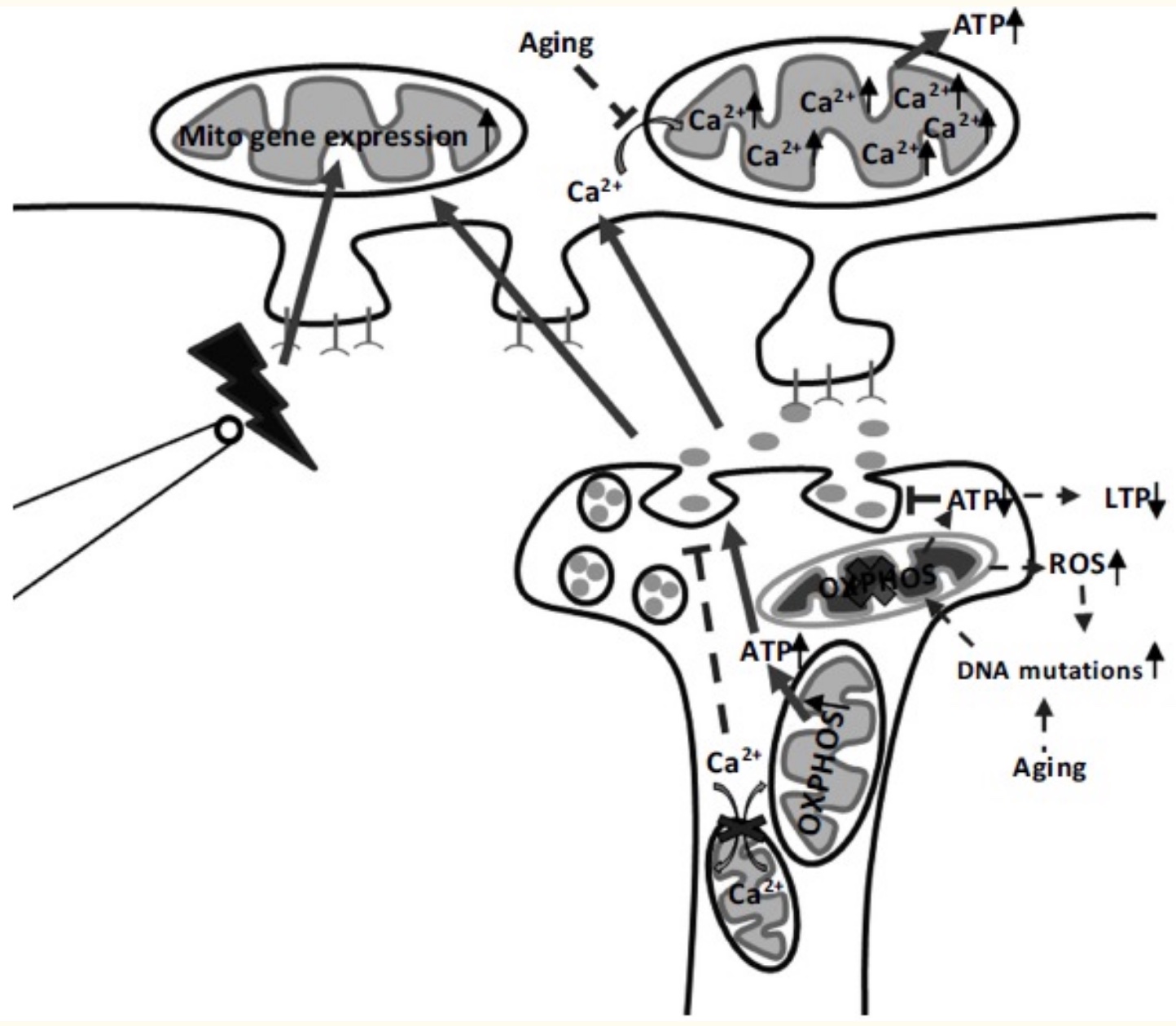
Too much calcium in the cytosol of the cells triggers mitochondria to take up the excess. This opens up pores in the mitochondrial membrane that can result in mitochondrial death and possibly cell death. This is what happens in injury or during hypoxic events, such as a heart attack.[ref]
Back to mitochondrial dysfunction and depression…
The link between mitochondrial dysfunction and depression has been shown in a lot of studies. Let’s take a look at a few animal studies as examples:
Adding stress hormones causes mitochondrial dysfunction:
The addition of corticosterone in mice will cause depression, and this is one way of making a mouse model of depression. To find out why, researchers tested the liver cells to see what had changed in the depressed mice. The results showed that the changes in gene expression were associated with decreased lipid metabolism (mitochondria using lipids less for fuel) and alterations in the genes needed for energy production in the mitochondria.
Adding healthy mitochondria eradicates depression symptoms:
Transplanting healthy mitochondria into the brains of mice cures depressive symptoms. In the experiment, the researchers caused depression in mice with lipopolysaccharide (a bacterial particle that revs up inflammation). The researchers assessed the depressive symptoms, looked at BNDF and neurogenesis in the brain, and quantified the neuroinflammation and oxidative stress. Injecting healthy mitochondria into the brain was able to reverse all the problems.[ref]
Targeted reversal of oxidative stress alleviates depression symptoms:
Animal studies also show that targeting mitochondrial oxidative stress via increasing SIRT3 levels reverses depression via reducing oxidative stress.[ref]
Direct link to brain plasticity:
Researchers used primates to show that malformed and misshapen mitochondria result in fewer synaptic contacts in the brain.[ref]
Brain energy, mood, and neurocognitive function:
The brain uses more energy than any other organ in the body. While small in size, your brain uses about 20% of the calories you eat.[ref]
A cool statistic: Each neuron in the cortex uses 4.7 billion ATP per second.[ref]
Like in the rest of the body, the mitochondria produce energy in brain cells. Here is a graphical representation of what is going on in a mitochondrion in depression.
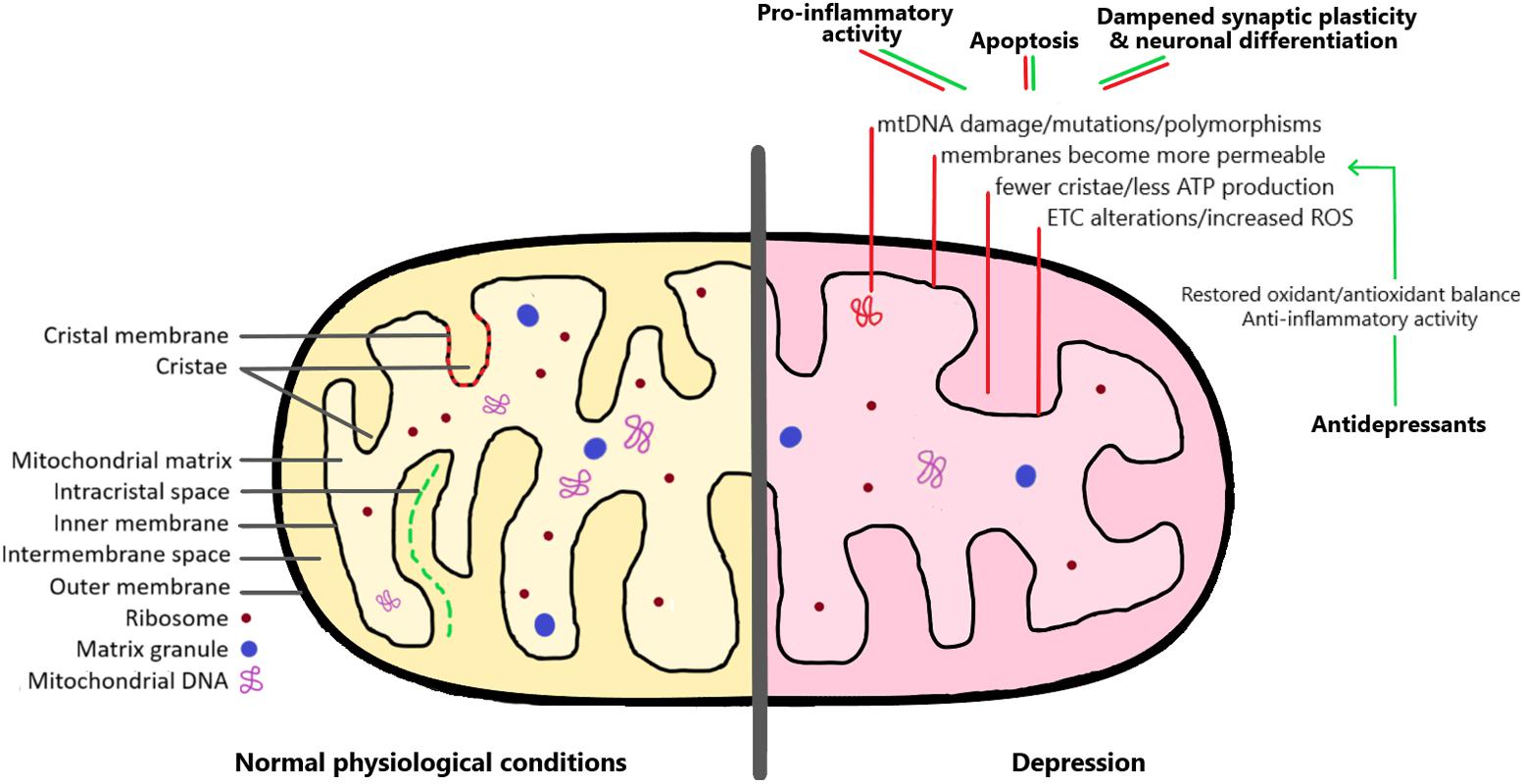
Decreased energy production in the brain leads to decreased neurogenesis in the hippocampus – and researchers hypothesize that this is the cause of long-term depression. In patients who have major depressive disorder, there is a decrease in the physical size of the hippocampus. Animal models of depression show a decrease in cell proliferation (creation of new brain cells) as well as an increase in cell death (apoptosis).[ref]
A relative lack of ATP due to mitochondrial dysfunction could also impact the ability of neurotransmitters to bind to their receptors. For signals to be sent from neuron to neuron, not only does the neurotransmitter have to be released into the synapse, but it also has to be received in a receptor, which then sets off a chain of events to send on the signal.
While that sounds cut-and-dried and makes a lot of sense, I want to note that not all studies agree and show decreases in brain volume and plasticity in depression. This could mean multiple physical changes that happen in depression (likely) – or – it could mean that some of the research is wrong and/or that I’m not understanding it correctly (also likely).
Let’s take a look at a few studies in humans on what happens when the brain doesn’t get enough energy:
- A proteomics study investigated proteins found in the brains of people with major depressive disorder compared with normal controls (all post-mortem). The biggest difference found in protein expression in depressive brains was in the proteins involved in energy metabolism.[ref]
- Researchers can’t look at mitochondrial function inside the brains of living people very easily. (I wouldn’t volunteer for that kind of study!) Researchers can look at muscle biopsies, though, to see what is happening to mitochondrial function there. In people with major depressive disorder and a chronic physical condition, mitochondrial ATP production decreases in muscle cells.[ref]
- Another post-mortem study on the brains of patients with depression, bipolar, or schizophrenia showed a specific mitochondrial protein (NDUFS7) involved in ATP production decreased significantly in patients with bipolar disorder.[ref]
Ketogenic diet:
A ketogenic diet is very low in carbohydrates, which causes a shift in the way that mitochondria produce energy. Instead of starting with glucose, ketosis causes a switch to using ketones derived from fat.
A number of studies and trials now show that a ketogenic diet works to decrease depression scores in many people. One study noted that the ketogenic diet improved mitochondrial function along with neurotransmitter balance.[ref][ref][ref]
Genes and mitochondrial dysfunction in depression:
For almost all diseases, genetic variants that are statistically linked to the disease will point toward the underlying cause. If mitochondrial dysfunction is a root cause of depression for some people, then it follows that there should be genetic variants in mitochondrial-related genes that are linked to depression.
It turns out that when researchers stopped focusing solely on the neurotransmitter genes, like the serotonin receptors, a clear picture emerged showing depression linked to circadian rhythm, inflammation, and mitochondrial function – perhaps with the mitochondrial function being at the root of long-term depressive disorders.
The mitochondria have their own genome, separate from what is in the nucleus of the DNA. The mitochondrial genome (mtDNA) is small, encoding only about 37 genes. These mtDNA genes are vital to mitochondrial function, but many genes in the nuclear DNA are also important for the mitochondria to work properly.
You have multiple mitochondria in each cell (sometimes thousands of mitochondria). Most of those mitochondria will have identical DNA – called homoplasmy. But some mitochondria may have mutations in the mtDNA. This alteration or mutation of the mtDNA is referred to as heteroplasmy. The higher the rate of heteroplasmy, the higher the incidence of disease, including depression.
A study in adults aged 70 and older showed increased heteroplasmy linked to an increased depression score.[ref]
Rare mitochondrial mutations that cause mitochondrial diseases are also linked to major depressive disorder.[ref] In fact, one study of people with hereditary (genetic) mitochondrial diseases showed that 54% had major depressive disorder and 17% had bipolar disorder.[ref]
Outside of the mitochondrial genome, genes in the nuclear genome that code for proteins in the mitochondria are also linked to depression. These genes include:[ref]
- TOMM40, which encodes a channel-forming protein that is essential for mitochondrial function.
- MTHFD1L, which codes for an enzyme used in the mitochondria in the creation of an important folate pathway metabolite. In addition to being important to the whole cell for folate metabolism, it is essential for maintaining mitochondrial function in the mitochondria. Decreased MTHFD1L directly decreases energy production in the mitochondria.
- ATP6V1B2, encodes an ATP enzyme that is important in the creation of proton gradients across membranes. The gene was identified in several genome-wide association studies as being important in major depressive disorder.
- MAO enzymes are important in the rate of breakdown of several important neurotransmitters, and MAO inhibitors are used as a type of antidepressant. In addition to their role in modulating neurotransmitter expression, MAOA and MAOB are found on the outer membrane of mitochondria. When overexpressed, MAO causes an excess of ROS in cells that injures mitochondria.[ref]
Related article: MAO-A and MAO-B: Neurotransmitter levels, genetics, and studies
- SOD2 encodes an enzyme that converts ROS (reactive oxygen species) in the mitochondria. This reduces or eliminates oxidative stress. Genetic variants decreasing SOD2 activity have links to depression.
- WFS1 encodes a protein found in neurons, and dysregulation of WFS1 leads to mitochondrial dysfunction and impaired calcium signaling. Genetic mutations that decrease WFS1 are linked to depression, psychiatric disorders, and suicide risk.[ref][ref][ref]
This is just the tip of the iceberg in terms of genes that can impact mitochondrial function.
Antidepressants and Oxidative Stress:
You may be wondering: “What about antidepressants?”. It turns out that some antidepressants act on oxidative stress.
Fluoxetine reverses the effects of oxidative stress in the mitochondria (animal study).[ref]
Tricyclic antidepressants (imipramine and amitriptyline) decreased mitochondrial disturbance and restored mitochondrial function in glioblastoma cells.[ref]
Mitochondria and Depression Genotype Report:
Lifehacks:
Talk with your doctor or health care provider if you have any questions before starting a supplement or changing your diet/lifestyle.
Diet and lifestyle suggestions:
Dumping the fast food and junk food:
Burning fat increases oxidative stress creation in the mitochondria.[ref] Generally, a Western diet that includes fried foods, like french fries and chicken nuggets, will increase oxidative stress. If you are eating a lot of junk food, this is the first place to target. Cut out the donuts, french fries, and pizza…
Increasing folate for MTHFD1L:
Increasing folate may help reduce homocysteine levels for people with MTHFD1L variants. Check out the folate-rich foods and recipes article.
Related Articles and Topics:
Key Genes to check for Alcoholism
Are there key genes to check for alcoholism? Learn more about the genetic connections to alcohol addiction and what research-backed treatment options are available.
Resilience: Genetic Variants Involved in Surviving Childhood Trauma
Exposure to childhood trauma, such as exposure to abuse, violence, or repeated stress, can have a long-lasting effect. Adults exposed to childhood trauma have higher rates of depression, PTSD, suicide, and anxiety disorders.
The Warrior Gene: Understanding the Role of Monoamine Oxidase Enzymes (MAOA and MAOB)
The MAOA and MAOB genes encode enzymes that break down certain neurotransmitters. People with low MAO may be prone to mood issues in certain circumstances.
Genetics and Anxiety
Did you know that about 1 in 5 people will deal with an anxiety disorder at some point in life? From generalized anxiety to separation anxiety to panic disorder – there are underlying physiological and genetic factors involved.