Key takeaways:
~ Alzheimer’s disease is a real risk for many people as they age. It is the most common form of dementia, affecting almost 1 in 3 people by the end of life.
~ One big change we have all been subjected to is the explosion of electronics and light at night.
~ Circadian rhythm, sleep, and melatonin are integral parts of preventing dementia.
Alzheimer’s risk, Alzheimer’s prevention:
With the advent of consumer genetic testing from 23andMe, AncestryDNA, etc, it is now easy to know if you are at a higher risk of getting Alzheimer’s Disease (AD). Those with APOE ε3 are at normal risk for Alzheimer’s, and those who carry an APOE ε4 allele (or two) are at an increased risk.
This is a touchy subject for some people, so please think it through before you check to see your APOE type. If you want to know, head to this page to check your raw data for APOE genotype.
Even without the APOE ε4 allele, Alzheimer’s and other forms of dementia are a real risk as we age, and the time to start with prevention of brain changes is in middle age.
Alzheimer’s Prevention:
Billions of dollars have been spent in the last couple of decades on trying to find drugs to stop the tangled accumulation of beta-amyloid plaque without much success. A new direction of research is looking into the ties between circadian rhythm dysfunction and Alzheimer’s disease.
I find this intriguing since the sudden increase in Alzheimer’s disease rate over the past few decades correlates with increasing chronic exposure to blue light at night, a circadian rhythm disruptor.
It has long been known that circadian disruption is a part of AD. Called ‘sundowning’, Alzheimer’s patients often are more active or confused in the evening /night and sleepy during the daytime.
Does Circadian Disruption Contribute to Alzheimer’s Risk?
The chicken-or-egg question:
Is Alzheimer’s caused by changing circadian rhythms –or– is the circadian dysfunction being caused by the disease?
A recent animal study looked at this question. The study used a mouse type that is bred to accumulate amyloid-beta and to also have a disruption of one of the core circadian genes, BMAL1. The results of the study indicate that disrupting the core circadian gene causes increased amyloid-beta production and plaque deposits. From the conclusion: “Our results demonstrate that loss of central circadian rhythms leads to disruption of daily hippocampal interstitial fluid Aβ oscillations and accelerates amyloid plaque accumulation, whereas loss of peripheral Bmal1 in the brain parenchyma increases expression of Apoe and promotes fibrillar plaque deposition.”[ref]
Yep, this is just a mouse study and needs to be replicated in humans. But holy cow – what if the increase in blue light at night, which directly acts to regulate BMAL1, is what is driving the rapid increase in Alzheimer’s disease?
Related Article: BMAL1’s impact on the “Big 3”: heart disease, cancer, and diabetes
So this led me to dive into other studies on AD and circadian rhythm. There are a lot of studies and reviews looking at the connection between circadian rhythms and AD, but, as a 2017 review concluded, there is still a lot to learn about how and when circadian dysfunction interacts with the pathogenesis of AD.[ref]
Is sleep disruption a risk factor for Alzheimer’s disease?
A 2015 article in the journal Nature does an excellent job of summing up the research up to that time. The article brings up the research on disrupted sleep being an additive risk factor along with APOE e4. Experiments have shown that amyloid-beta levels rise and fall in a daily circadian pattern, both in mice and humans.
The study goes on to say (emphasis mine): “Sleep deprivation exacerbates Aβ plaque pathology while enhancing sleep by inhibiting orexin signaling attenuates plaque accumulation. Finally, sleep deprivation exerts a variety of Aβ-independent effects in the brain that could exacerbate neurodegeneration. Because relatively small alterations in Aβ levels can translate into considerable changes in plaque pathology over a long timeframe, chronic mild sleep disturbances throughout life might conceivably facilitate Aβ deposition, setting in motion a feed-forward cycle in which Aβ pathology, in turn, impairs the sleep-wake cycle.” You can read the full Nature article here: https://www.nature.com/articles/emm2014121
A study that came out in Feb. 2018 looked at how the APOE ε4 genotype interacts with sleep cycles. It concluded: “Our findings suggest that the APOE ε4 allele may act as a moderator in the relationship between the sleep-wake cycle and Aβ accumulation in CN [cognitively normal] older adults.“[ref]
Another study that came out in January 2018 used an animal model of AD with tau accumulation. The study found that dim light at night caused disrupted circadian rhythms and neurodegeneration.[ref]
More research from 2017 using an animal model expressing the human tau protein found: “we demonstrate that sleep can be used as a therapeutic to reverse deficits that accrue during the expression of toxic peptides associated with Alzheimer’s disease.”[ref]
Is melatonin linked to Alzheimer’s?
Mouse studies have shown that melatonin specifically acts to decrease amyloid-beta accumulation by inhibiting the β-amyloid precursor protein.[ref] Melatonin levels decrease as we get older, with a lot of adults having limited or no melatonin production via the pineal gland by age 85. Coincidentally, this is around the same age that Alzheimer’s risk skyrockets?
Some researchers have focused on mitochondria and energy production in the brain as a root cause of Alzheimer’s. Other researchers point towards oxidative stress in the cells (which goes along with unhealthy mitochondria). All of these theories come together, though, with a focus on blocking light at night in order to increase melatonin levels. Melatonin acts within cells as a potent antioxidant, decreasing oxidative stress and increasing healthy mitochondria in the brain.[ref]
In fact, another recent study used mice that had been given a genetic mutation to cause early-onset familial Alzheimer’s disease. The study found that giving those mice melatonin increased mitochondrial biogenesis, decreased memory deficits, increased spatial learning, and decreased amyloid-beta deposition.[ref]
Related Article: Melatonin: Key to Health and Longevity
Lifehacks:
So what can you do with this information linking circadian rhythm dysfunction to AD? First, take a real look at your sleep quality and sleep environment. Second, it may be time to make some lifestyle changes to limit blue light at night.
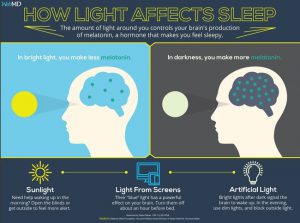
Sleep in a dark room:
The study on dim light at night is a good reminder to make sure that you are sleeping comfortably in a really dark room. Put a piece of electrical tape over all the annoying little LED lights (or unplug them). If you need a clock in your bedroom, go with an automatically dimming clock that is red. And most importantly, put up some blackout curtains. It makes a huge difference in sleep to actually sleep in true darkness.
Block blue light at night for good sleep:
Why block the blue-light wavelengths before bed? Prior to the advent of electric lights, all we had for thousands of years was candlelight or fire to light up the night. Then came the incandescent bulb, which has only a small amount of light coming from the shorter blue wavelengths. Finally, we all got color TVs, LED or CFL light bulbs, and smartphones or tablets, all of which beam light in the short blue wavelengths at us in the evenings.
Our core circadian rhythm is reset each day with 480 nm wavelength (blue light). Humans used to get up each morning, go outside and reset their circadian rhythms with the light coming from the sun.
Now, we are exposing our eyes well into the night to the specific wavelengths that indicate daytime. Circadian mismatch. We, humans, are resilient and can handle circadian disruption for a time, but the chronic and pervasive bombardment of blue wavelength light at night is now linked to increased risk of cancer, heart disease, mood disorders, obesity, Alzheimer’s, and diabetes.
Specifically, light in the blue wavelengths blocks melatonin production. This is what is supposed to happen each morning, but now we are shutting down melatonin production at night when it is supposed to rise dramatically.
There is a great Popular Mechanics article that shows the wavelengths of different bulbs including CFL, LED, and incandescent.
Should you wear blue-blocking glasses at night?
Before rocking the blue-blocking glasses in the evening, I would have told you that I slept well. And I did usually fall asleep easily…staying asleep all night was sometimes a problem, though. But I had no idea what good sleep was until I took the plunge and bought a cheap $10 pair of dorky-looking blue-blocking safety glasses. I’ve now upgraded to some that look a little better and cost in the $20-$30 range. Still not expensive when it comes to preventing Alzheimer’s!
I’ve gotten differing feedback from friends who I’ve talked into trying the blue-blocking glasses at night. A couple of friends had the same reaction that I had: incredible sleep, improved mood in the morning (almost giddy at first), and no plans to ever stop wearing the blue blockers. On the other hand, other friends and family have really struggled with giving the glasses a shot. They find that they need to take them on and off to put on reading glasses, or they need to take them off while taking out contacts. All in all, they aren’t seeing enough benefit to regularly wear the glasses, which may come from the intermittent light negating the effect.
Check out my Review of Blue-blocking Glasses for more information and different options available.
If you really hate blue-blocking glasses, the alternative is to shut off the blue light at night. Turn off your electronics (TV, phone, laptop, tablet) a couple of hours before bed. And then turn off the bright overhead lights and switch on some lamps that use either low-watt incandescent or antique candle-style bulbs.
Related Articles and Genes:
Alzheimer’s and APOE genotype
One very important gene that has been extremely well researched for Alzheimer’s disease is the APOE gene. This gene is involved in carrying cholesterol and other fats in your bloodstream, and a common variant of the gene is linked to a higher risk of Alzheimer’s.
Genetic Mutations that Protect Against Alzheimer’s Disease
Alzheimer’s disease is a scary possibility that faces many of us today — whether for ourselves or for aging parents and grandparents. Currently, 10% of people aged 65 or older have Alzheimer’s disease (AD). It is a disease for which prevention needs to start decades before the symptoms appear.
Circadian Rhythms: Genes at the Core of Our Internal Clocks
Circadian rhythms are the natural biological rhythms that shape our biology. Most people know about the master clock in our brain that keeps us on a wake-sleep cycle over 24 hours. This is driven by our master ‘clock’ genes.
Sleep Report: Genetic Causes of Sleep Problems
A quick overview of how your genetic variants impact various aspects of sleep including insomnia, circadian rhythm, and sleep quality.
Serotonin 2A receptor variants: psychedelics, brain aging, and Alzheimer’s disease
Learn why tiny doses of LSD are being studied for Alzheimer’s- and other ways that you can target the serotonin 2A receptor.
Lithium for Preventing Alzheimer’s Diasease
Find out what the latest research on low-dose lithium shows for Alzheimer’s prevention.
References:
APOE – SNPedia. https://www.snpedia.com/index.php/APOE. Accessed 21 Sept. 2021.
Dissel, Stephane, et al. “Enhanced Sleep Reverses Memory Deficits and Underlying Pathology in Drosophila Models of Alzheimer’s Disease.” Neurobiology of Sleep and Circadian Rhythms, vol. 2, Jan. 2017, pp. 15–26. PubMed, https://doi.org/10.1016/j.nbscr.2016.09.001.
Herrman, John. “PM’s Ultimate Light Bulb Test.” Popular Mechanics, 20 Sept. 2011, https://www.popularmechanics.com/technology/gadgets/tests/incandescent-vs-compact-fluorescent-vs-led-ultimate-light-bulb-test.
Hwang, Jeong Yeon, et al. “Moderating Effect of APOE Ε4 on the Relationship between Sleep-Wake Cycle and Brain β-Amyloid.” Neurology, vol. 90, no. 13, Mar. 2018, pp. e1167–73. PubMed, https://doi.org/10.1212/WNL.0000000000005193.
Kim, Mari, et al. “Short-Term Exposure to Dim Light at Night Disrupts Rhythmic Behaviors and Causes Neurodegeneration in Fly Models of Tauopathy and Alzheimer’s Disease.” Biochemical and Biophysical Research Communications, vol. 495, no. 2, Jan. 2018, pp. 1722–29. PubMed, https://doi.org/10.1016/j.bbrc.2017.12.021.
Musiek, Erik S. “Circadian Rhythms in AD Pathogenesis: A Critical Appraisal.” Current Sleep Medicine Reports, vol. 3, no. 2, June 2017, pp. 85–92. PubMed, https://doi.org/10.1007/s40675-017-0072-5.
—. “Sleep, Circadian Rhythms, and the Pathogenesis of Alzheimer Disease.” Experimental & Molecular Medicine, vol. 47, no. 3, Mar. 2015, pp. e148–e148. www.nature.com, https://doi.org/10.1038/emm.2014.121.
Salamon, Maureen. “Sleep and Blue Light.” WebMD, https://www.webmd.com/sleep-disorders/sleep-blue-light. Accessed 21 Sept. 2021.
Shukla, Mayuri, et al. “Mechanisms of Melatonin in Alleviating Alzheimer’s Disease.” Current Neuropharmacology, vol. 15, no. 7, Oct. 2017, pp. 1010–31. PubMed Central, https://doi.org/10.2174/1570159X15666170313123454.
Song, ChaoYuan, et al. “Mitochondrial Biogenesis Mediated by Melatonin in an APPswe/PS1dE9 Transgenic Mice Model.” Neuroreport, vol. 29, no. 18, Dec. 2018, pp. 1517–24. PubMed, https://doi.org/10.1097/WNR.0000000000001139.
—. “Mitochondrial Biogenesis Mediated by Melatonin in an APPswe/PS1dE9 Transgenic Mice Model.” Neuroreport, vol. 29, no. 18, Dec. 2018, pp. 1517–24. PubMed, https://doi.org/10.1097/WNR.0000000000001139.
Untitled. https://rupress.org/jem/article/215/4/1059/42402/Regulation-of-amyloid-dynamics-and-pathology-by. Accessed 21 Sept. 2021.

