Key takeaways:
~ The APOE alleles are important in understanding your Alzheimer’s risk, and the data is found in your 23andMe raw data file (if you want to know).
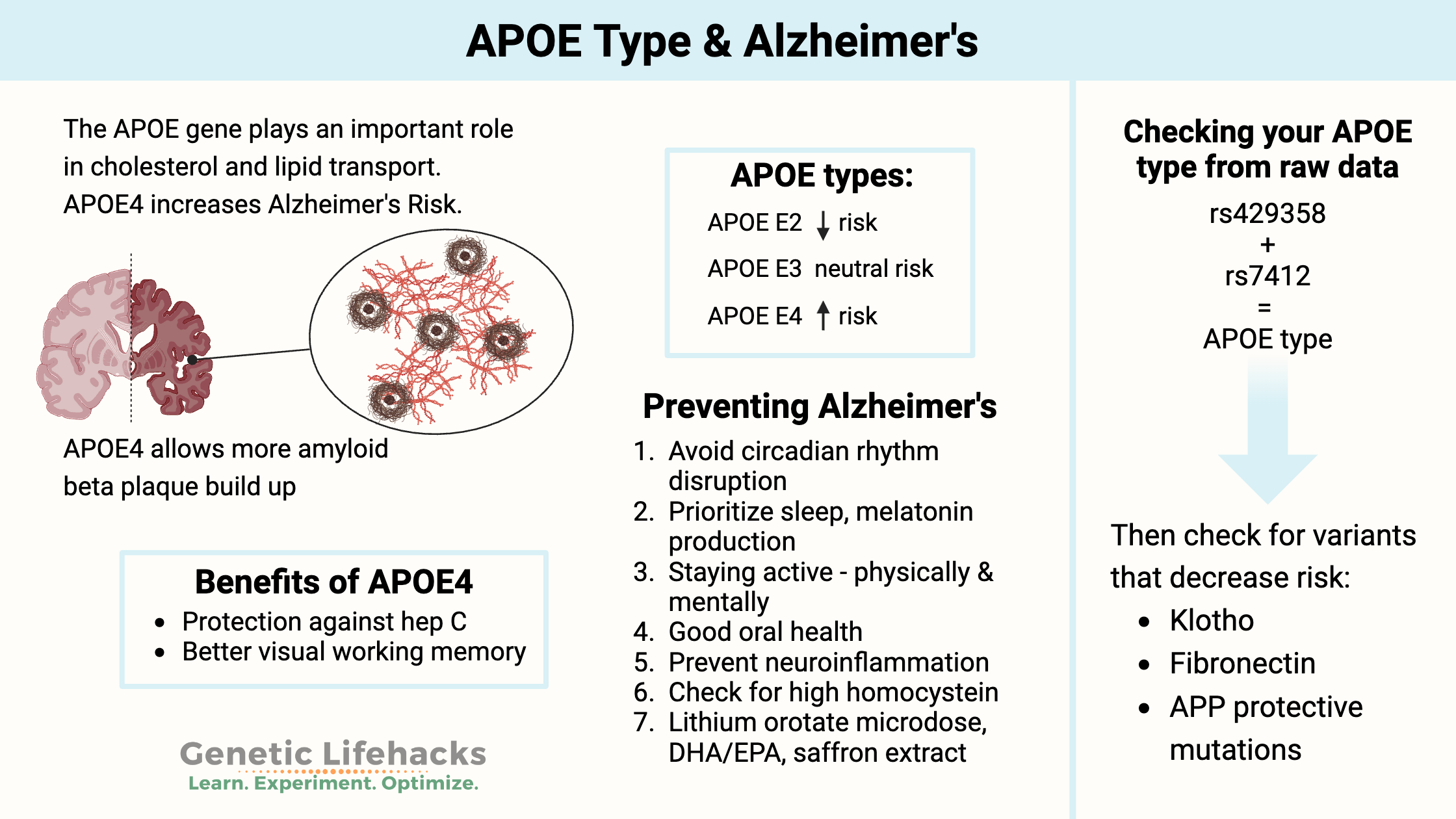
~ APOE is involved in carrying cholesterol and other fats in your bloodstream, and a common variant of the gene is strongly linked to a higher risk of Alzheimer’s.[ref]
~ Alzheimer’s risk is influenced both by genes and environmental factors. Genes are only one part of the equation for Alzheimer’s.
~ Knowing your risk can help you prioritize lifestyle changes for the prevention of Alzheimer’s disease.
APOE Gene Variants:
Your APOE type is defined as a combination of three different alleles (ε2, ε3, or ε4), and you will have one APOE allele from each parent.
The NIH website explains:
| APOE Allele | Effect on Alzheimer’s Risk |
|---|---|
| ε2 | Decreased risk (protective) |
| ε3 | Neutral (normal risk) |
| ε4 | Increased risk (>40% of Alzheimer’s cases carry this allele) |
What does APOE do?
APOE (apolipoprotein E) is important in the metabolism and transport of cholesterol and other fats in the body, particularly in the central nervous system.
The APOE protein is primarily involved in the transport and metabolism of lipids in the blood, where it helps to carry cholesterol and other lipids to and from cells, and in the brain, where it helps to transport lipids across the blood-brain barrier.
In the brain, APOE helps to regulate the clearance of beta-amyloid protein, which is a key component of the plaques that form in the brains of people with Alzheimer’s disease. APOE is also involved in many other processes in the body, such as inflammation, immune function, and repair of tissues after injury.
The different APOE genotypes (E2, E3, E4) result in different-sized APOE molecules. Additionally, the different APOE types are associated with the level of plasma APOE.
The APOE E2 allele is linked to higher plasma APOE levels, while the APOE E4 allele is linked to lower plasma APOE levels. One study explained that “Low plasma levels of apoE are associated with increased risk of future Alzheimer’s disease and all dementia in the general population…”[ref]
Genotype report: Finding your APOE type
AncestryDNA NOTE: The initial AncestryDNA v2 data (e.g. 2015, 2016) commonly had errors in the APOE rs ids and was not reliably accurate. They have removed one of the APOE rs ids from their data set, and their current raw data files do not include the rs ids needed for determining APOE type.[ref] Other data files that work with Genetic Lifehacks membership, such as 23andMe, selfdecode, sequencing.com, or MyHeritage data files, should work fine, although rare errors are always possible.
To determine your APOE type from your 23andMe data or another source, you will need to look at the following two rs IDs: rs429358 and rs7412.
Check your genetic data for rs429358 (23andMe v4, v5):
- T/T: typical; look at this in combination with rs7412 (chart below)
- C/T: look at this in combination with rs7412 (chart below)
- C/C: look at this in combination with rs7412 (chart below)
Members: Your genotype for rs429358 is —.
Check your genetic data for rs7412 (23andMe v4, v5):
- C/C: typical; look at this in combination with rs429358 (chart below)
- C/T: look at this in combination with rs429358 (chart below)
- T/T: look at this in combination with rs429358 (chart below)
Members: Your genotype for rs7412 is —.
| rs429358 | rs7412 | APOE Allele | Alzheimer’s Risk |
|---|---|---|---|
| Your genotype: — | Your genotype: — | ||
| T/T | T/T | ε2/ε2 | lowest risk (rare)
🚩 This is likely your genotype, but double check that this automated note is correct
|
| T/T | C/T | ε2/ε3 | lower risk
🚩 This is likely your genotype, but double check that this automated note is correct
|
| C/T | C/T | ε2/ε4 | slightly higher risk than normal
🚩 This is likely your genotype, but double check that this automated note is correct
|
| T/T | C/C | ε3/ε3 | normal risk
🚩 This is likely your genotype, but double check that this automated note is correct
|
| C/T | C/C | ε3/ε4 | higher risk
🚩 This is likely your genotype, but double check that this automated note is correct
|
| C/C | C/C | ε4/ε4 | highest risk[ref]
🚩 This is likely your genotype, but double check that this automated note is correct
|
Video explanation of how to read the chart
APOE types:
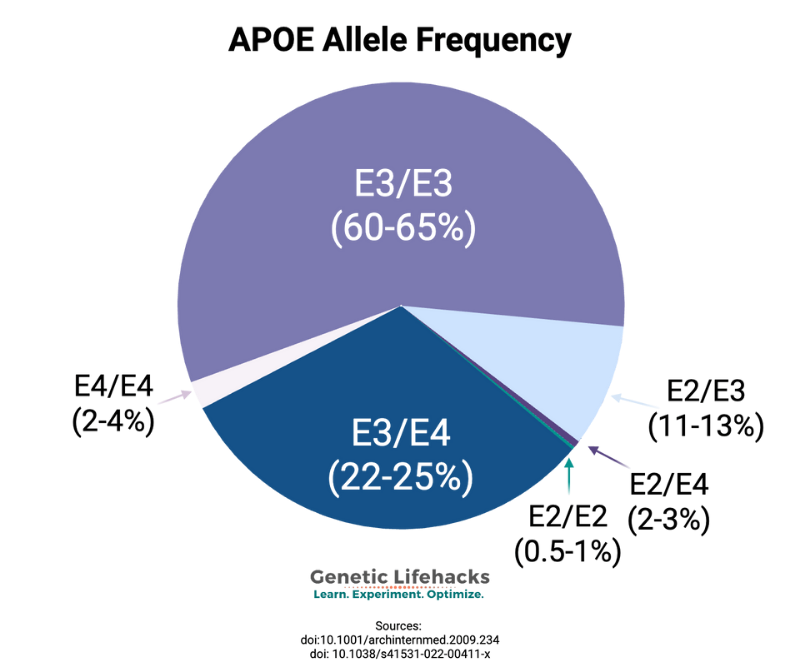
You inherit one APOE allele from your mother and one from your father. Thus, you will have two APOE allele types. There are six possible combinations (listed above): E2/E2, E2/E3, E3/E3, E3/E4, E4/E4, E2/E4.
E3/E3: The APOE E3 type is the normal and most common type. People with two copies of APOE E3 will have what is considered the typical risk allele for Alzheimer’s disease.
E2/E3: APOE2 can be protective against Alzheimer’s disease. This combination decreases the risk of Alzheimer’s disease significantly.
E2/E4: If you’re heterozygous at both SNPs (CT at each), standard interpretation is ε2/ε4. Theoretically, a rare ε1 haplotype could complicate this, but it is really rare. Studies vary on whether the E2/E4 combination is similar in risk to E3/E3, slightly elevated, or slightly decreased. A recent study showed that the E2/E4 combination had significantly reduced amyloid-beta levels compared to E3/E4.[ref]
What does APOE E4 mean?
The APOE E4 genotype elevates the risk of developing Alzheimer’s disease compared to the more common version of the gene, APOE3. The APOE4 allele also increases the risk of developing the disease at a slightly younger age.[ref]
In addition to Alzheimer’s disease, the APOE4 allele has also been linked to an increased relative risk of other neurodegenerative conditions, including Lewy body dementia and TDP-43 pathology in Alzheimer’s disease. Additionally, APOE E4 carriers are at a slightly increased relative risk of stroke and cardiovascular disease.[ref][ref][ref]
Other factors involved in Alzheimer’s:
Again, your APOE gene isn’t the only factor involved in getting Alzheimer’s Disease.
- A minority of people with the E4/E4 alleles don’t end up with Alzheimer’s, but since some do not end up with Alzheimer’s, APOE is not completely penetrant.
- Environmental and lifestyle factors play a big role in who gets Alzheimer’s. (Covered in the Lifehacks for Alzheimer’s Prevention section below)
- Other genes could add to or decrease your risk.
- Check out my article on genetic mutations that decrease the risk of Alzheimer’s.
- Variants in the KLOTHO gene can decrease the APOE E4 elevated risk.
- A new research study found fibronectin variants are protective against Alzheimer’s in APOE4 individuals.
- New research has found that exposure to HSV-1 can contribute to Alzheimer’s risk and progression. [ref][ref]
- In people with Alzheimer’s disease, there is reduced blood glucose uptake and energy (ATP) creation in the brain. Glucose enters the brain through glucose transporters in the blood-brain barrier. Brain glucose is tightly regulated.[ref]
- A 2025 pilot study found that 20g/day of supplemental creatine was well tolerated by patients with Alzheimer’s, and the researchers hypothesized that this may increase cognitive performance in Alzheimer’s. [ref]
- Check out my article on creatine and cognitive performance
Thus, your APOE type isn’t the complete picture, but research does show it to be the most significant common genetic risk factor for Alzheimer’s disease.
APOE E4 benefits:
For almost every genetic variant with a negative impact common in the population, there is also a tradeoff or benefit. The reason that the allele is common is usually due to positive selection for the genotype.
This holds true even for the APOE E4 allele.
| Benefit | Description |
|---|---|
| Parasite Protection | May aid in clearing parasites and hepatitis C |
| Age-related Macular Degeneration Protection | Protective effect against macular degeneration |
| Visual Working Memory in Older Age | Better visual working memory at age 70 in some, especially at higher β-amyloid burden |
| Infectious Disease Protection | Lower risk of liver issues in hepatitis C |
| Obesity Paradox | Obesity protective vs. dementia in APOE E4, but risk in E2/E3 alleles |
Parasite protection:
Studies in the Amazon show that older adults with the APOE E4 allele, along with high parasite burdens, were cognitively better off than those with the E3 or E2 alleles. Viral hepatitis C, Giardia, and Cryptosporidium were cleared more easily.[ref]
Protective against age-related macular degeneration:
The APOE E4 allele has been shown in several studies to protect against macular degeneration.[ref]
Visual working memory benefits at age 70:
“ε4-carriers also recalled locations more precisely, with a greater advantage at higher β-amyloid burden. These results provide evidence of superior visual working memory in ε4-carriers, showing that some benefits of this genotype are demonstrable in older age, even in the preclinical stages of Alzheimer’s disease.”[ref]
Infectious disease protection:
The APOE E4 allele is linked to a decreased risk of chronic hepatitis C and a lower risk of liver problems in people who have hepatitis C. [ref]
Obesity Paradox for APOE and Dementia:
People who are obese have been shown in studies to be at a greater risk of dementia, but not all studies show this result. It turns out that the APOE allele may be the difference.
A recent study showed that people with APOE E2 and APOE E3 alleles are at a greater risk of cognitive decline or dementia if they are obese. However, obesity was protective against dementia and Alzheimer’s when combined with the APOE E4 allele.[ref]
Diagnosis of Alzheimer’s disease:
One problem with Alzheimer’s disease is that it is not a clear-cut diagnosis. There are many different causes of dementia, and historically, the only way to know for sure that it was Alzheimer’s was through autopsy after death. While there are new biomarkers being looked at for diagnosis (none definitive yet), keep in mind that if you have a “family history” of Alzheimer’s that even recent studies show that up to 23% of diagnoses were likely incorrect.[ref]
What else causes the symptoms of Alzheimer’s disease? Medications, including sleeping aids (benzodiazepines), narcotic pain relievers, antidepressants, anticholinergics, statins, warfarin, and corticosteroids, can all give some people memory and cognitive function problems that are similar to mild Alzheimer’s disease. [ref]
Lifehacks: Alzheimer’s Prevention
If you are at an increased risk for Alzheimer’s, the key is to use this knowledge to do all that you can to decrease your risk.
Download my free eBook on Alzheimer’s prevention
Research on Alzheimer’s prevention:
Here’s an overview of prevention strategies, with details below:
| Strategy | Evidence/Notes |
|---|---|
| Block blue light at night | Supports healthy melatonin/circadian rhythm, linked to prevention |
| Maintain physical activity | Exercise shown to lower risk |
| Brain energy support (cell energetics) | Targeting glucose/prostaglandin-E2 pathways shows promise in mice |
| Saffron extract | As effective as standard AD drugs in trials |
| Low-dose lithium | Epidemiological & clinical trial support for protection |
| Lemon balm extract | Some evidence for improved cognition in mild/moderate AD |
| Support liver health | TUDCA & altered bile acid profile in studies |
| Use NSAIDs (cautiously) | Population studies show reduced risk, but risk varies by genotype |
| Monitor cardiovascular health/homocysteine | High homocysteine = higher risk |
| Maintain healthy oral hygiene | P. gingivalis & oral microbiome linked to AD risk |
| Reduce saturated fat intake (APOE E4) | Some evidence this may help in E4 carriers |
| Evaluate hormone therapy | In APOE E4 women, HRT increases risk markers |
| Use of luteolin, DHA/EPA (fish oil) | Potential neuroprotective effects, variable by genotype |
| Minimize alcohol (APOE E4 carriers) | Alcohol increases cognitive decline risk in E4 |
Circadian Rhythm and Melatonin:
Number one on my list for preventing Alzheimer’s is blocking blue light at night to boost melatonin and help with circadian rhythm.
There is a ton of research showing a strong connection between circadian rhythm disruption, melatonin production, insulin regulation, and healthy brain aging.[ref][ref][ref][ref]
Our natural circadian rhythm causes melatonin to rise in the evening and stay elevated until morning. Light in the shorter, blue wavelengths signals through receptors in our eyes to turn off melatonin production in the morning.
However, our modern environment with electric lights at night, especially TVs and phones, disrupts our natural circadian rhythm.
You can either block blue light with 100% blue-light-blocking glasses or simply avoid electronics and bright overhead light for a couple of hours before sleep. Blue-blocking glasses, worn in the evening for several hours before bed, increase natural melatonin production by about 50% in just two weeks.[ref]
Read more about Light at Night and Alzheimer’s Risk. (in-depth on all the research on circadian rhythm)
What about supplemental melatonin?
Clinical trials are also evaluating the use of melatonin supplements for Alzheimer’s.[ref] Additionally, melatonin has been shown to positively reduce any increase in cardiovascular disease risk associated with the APOE E4 allele.[ref]
Read more about Supplemental Melatonin research studies and dosage details.
Brain energy and neurodegenerative diseases:
Alzheimer’s has been dubbed as “type 3 diabetes” by some researchers due to the changes in the way glucose is used in the brain. In addition to the decreased energy in the brain cells, an increase in inflammation is seen in Alzheimer’s and dementia patients.
A study in mice investigated the changes in metabolism in brain cells linked to Alzheimer’s and cognitive decline in aging. In this study, the researchers showed that energy production is reduced in microglia and macrophages in response to increased prostaglandin E2, an inflammatory signal. Specifically, prostaglandin E2 caused glucose to be stored as glycogen rather than being used for cellular energy production.
Most importantly, the study showed that inhibiting the EP2 receptor for prostaglandin E2 in myeloid cells was sufficient to increase cellular energetics. Blocking that prostaglandin EP2 receptor reversed cognitive aging in mice.[ref]
Saffron Extract:
Access this content:
An active subscription is required to access this content.
Related Articles and Topics:
Serotonin 2A receptor: Psychedelic response and Alzheimer’s disease
Alzheimer’s and Light at Night: Taking action to prevent this disease
References:
“Alzheimer’s Disease Genetics Fact Sheet.” National Institute on Aging, https://www.nia.nih.gov/health/alzheimers-disease-genetics-fact-sheet. Accessed 29 Apr. 2022.
APOE – SNPedia. https://www.snpedia.com/index.php/APOE. Accessed 29 Apr. 2022.
Bessone, Fernando. “Non-Steroidal Anti-Inflammatory Drugs: What Is the Actual Risk of Liver Damage?” World Journal of Gastroenterology : WJG, vol. 16, no. 45, Dec. 2010, pp. 5651–61. PubMed Central, https://doi.org/10.3748/wjg.v16.i45.5651.
Downer, Brian, et al. “The Relationship between Midlife and Late Life Alcohol Consumption, APOE E4 and the Decline in Learning and Memory among Older Adults.” Alcohol and Alcoholism (Oxford, Oxfordshire), vol. 49, no. 1, Feb. 2014, pp. 17–22. PubMed, https://doi.org/10.1093/alcalc/agt144.
Farrer, L. A., et al. “Effects of Age, Sex, and Ethnicity on the Association between Apolipoprotein E Genotype and Alzheimer Disease. A Meta-Analysis. APOE and Alzheimer Disease Meta Analysis Consortium.” JAMA, vol. 278, no. 16, Oct. 1997, pp. 1349–56.
in ’t Veld, Bas A., et al. “Nonsteroidal Antiinflammatory Drugs and the Risk of Alzheimer’s Disease.” New England Journal of Medicine, vol. 345, no. 21, Nov. 2001, pp. 1515–21. Taylor and Francis+NEJM, https://doi.org/10.1056/NEJMoa010178.
Jenwitheesuk, Anorut, et al. “Melatonin Regulates Aging and Neurodegeneration through Energy Metabolism, Epigenetics, Autophagy and Circadian Rhythm Pathways.” International Journal of Molecular Sciences, vol. 15, no. 9, Sept. 2014, pp. 16848–84. PubMed Central, https://doi.org/10.3390/ijms150916848.
Kim, Mari, et al. “Short-Term Exposure to Dim Light at Night Disrupts Rhythmic Behaviors and Causes Neurodegeneration in Fly Models of Tauopathy and Alzheimer’s Disease.” Biochemical and Biophysical Research Communications, vol. 495, no. 2, Jan. 2018, pp. 1722–29. PubMed, https://doi.org/10.1016/j.bbrc.2017.12.021.
Metformin | Cognitive Vitality | Alzheimer’s Drug Discovery Foundation. http://www.alzdiscovery.org/cognitive-vitality/condition/lithium-drug-treatments. Accessed 29 Apr. 2022.
Nunes, Marielza Andrade, et al. “Microdose Lithium Treatment Stabilized Cognitive Impairment in Patients with Alzheimer’s Disease.” Current Alzheimer Research, vol. 10, no. 1, Jan. 2013, pp. 104–07. PubMed, https://doi.org/10.2174/1567205011310010014.
Paterniti, Irene, et al. “Neuroprotection by Association of Palmitoylethanolamide with Luteolin in Experimental Alzheimer’s Disease Models: The Control of Neuroinflammation.” CNS & Neurological Disorders Drug Targets, vol. 13, no. 9, 2014, pp. 1530–41. PubMed, https://doi.org/10.2174/1871527313666140806124322.
Rovio, Suvi, et al. “Leisure-Time Physical Activity at Midlife and the Risk of Dementia and Alzheimer’s Disease.” The Lancet Neurology, vol. 4, no. 11, Nov. 2005, pp. 705–11. www.thelancet.com, https://doi.org/10.1016/S1474-4422(05)70198-8.
Seshadri, Sudha, et al. “Plasma Homocysteine as a Risk Factor for Dementia and Alzheimer’s Disease.” New England Journal of Medicine, vol. 346, no. 7, Feb. 2002, pp. 476–83. Taylor and Francis+NEJM, https://doi.org/10.1056/NEJMoa011613.
Shechter, Ari, et al. “Blocking Nocturnal Blue Light for Insomnia: A Randomized Controlled Trial.” Journal of Psychiatric Research, vol. 96, Jan. 2018, pp. 196–202. PubMed, https://doi.org/10.1016/j.jpsychires.2017.10.015.
Shi, Le, et al. “Sleep Disturbances Increase the Risk of Dementia: A Systematic Review and Meta-Analysis.” Sleep Medicine Reviews, vol. 40, Aug. 2018, pp. 4–16. PubMed, https://doi.org/10.1016/j.smrv.2017.06.010.
Shukla, Mayuri, et al. “Mechanisms of Melatonin in Alleviating Alzheimer’s Disease.” Current Neuropharmacology, vol. 15, no. 7, 2017, pp. 1010–31. PubMed, https://doi.org/10.2174/1570159X15666170313123454.
Wang, Jun, et al. “Anti-Inflammatory Drugs and Risk of Alzheimer’s Disease: An Updated Systematic Review and Meta-Analysis.” Journal of Alzheimer’s Disease: JAD, vol. 44, no. 2, 2015, pp. 385–96. PubMed, https://doi.org/10.3233/JAD-141506.
Zisapel, Nava. “New Perspectives on the Role of Melatonin in Human Sleep, Circadian Rhythms and Their Regulation.” British Journal of Pharmacology, vol. 175, no. 16, Aug. 2018, pp. 3190–99. PubMed, https://doi.org/10.1111/bph.14116.