Key takeaways:
~ Researchers have identified BPIFB4 gene variants in people who are long-lived.
~ BPIFB4 modulates immune response and impacts cardiovascular disease.
~ You can see if you have the BPIFB4 longevity variant in the genotype report below.
BPIFB4 is identified as a longevity gene:
Healthy longevity is a goal for many of us, and genes come into play here in several ways. In general, not having deleterious genetic variants is linked to a longer overall healthspan. For example, mutations linked to hereditary cancers are associated with a shorter healthspan, and a lack of cancer-related variants is linked to an average longer lifespan.
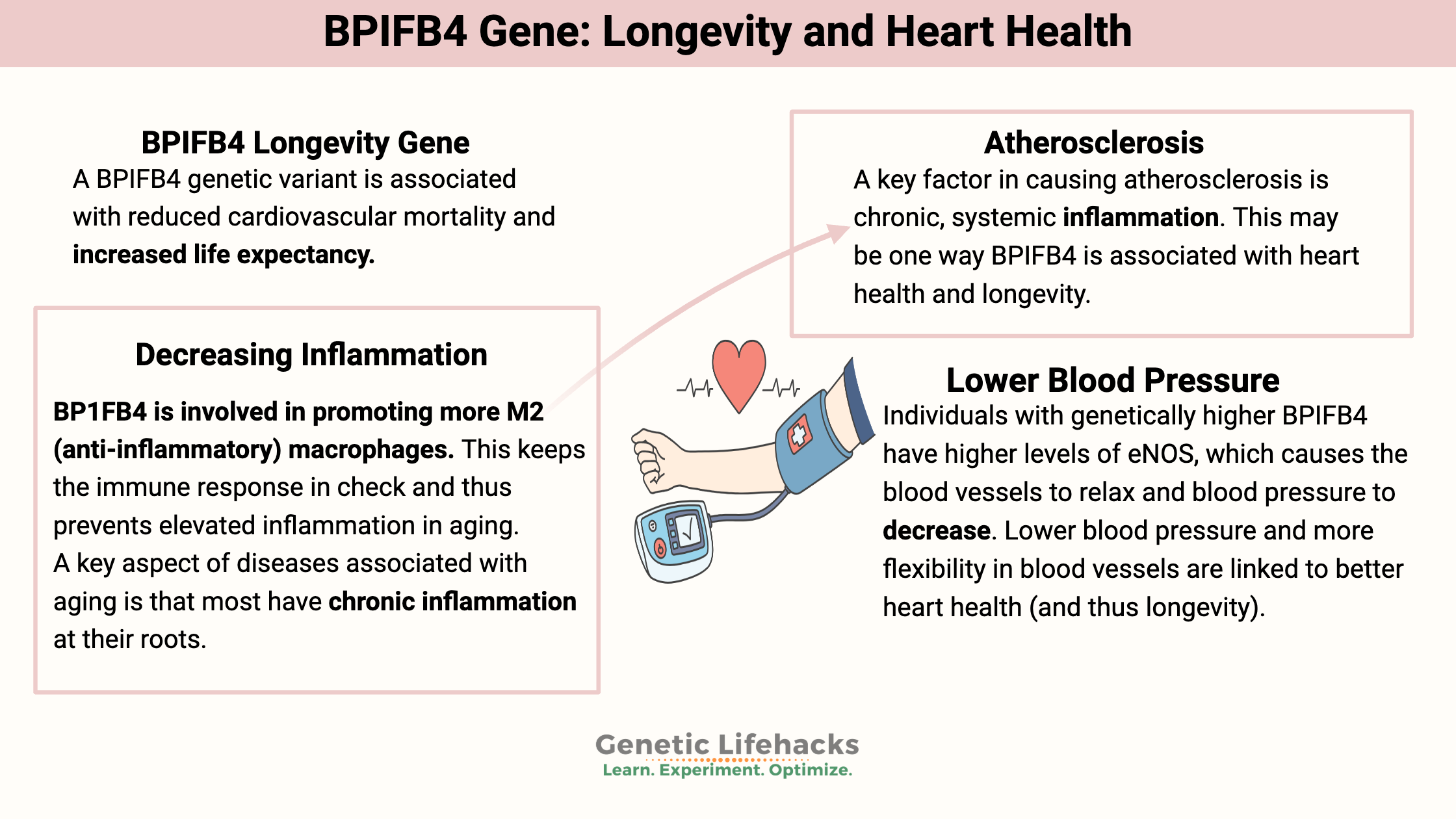
The most common cause of death is cardiovascular disease. It’s unsurprising that a genetic variant associated with reduced cardiovascular mortality is also associated with increased life expectancy on average.
What does BPIFB4 do?
The BPIFB4 gene encodes Bactericidal/Permeability-Increasing Fold-Containing Family B member 4. It was discovered to be linked to a longer lifespan in a genome-wide association study of long-lived individuals in Italy (over age 95). The discovery spurred research into what the BPIFB4 protein does in the body and whether it can be manipulated to extend healthspan.[ref][ref]
The research on BPIFB4 is all relatively new. Researchers have found that it is a secreted protein that modulates the immune response and keeps inflammation in check. BPIFB4 is found in respiratory secretions and circulates through blood vessels. It participates in response to airborne pathogens as well as having immunomodulatory properties.[ref]
Decreasing inflammation:
Macrophages are a type of immune cell that come in two forms:
- M1 macrophages are inflammatory, fighting off pathogens
- M2 macrophages balance out the immune response
While we need a robust immune response against pathogens, having too much M1 activation is linked to many chronic diseases, such as heart disease. A key aspect of diseases associated with aging is that most have chronic inflammation at their roots.
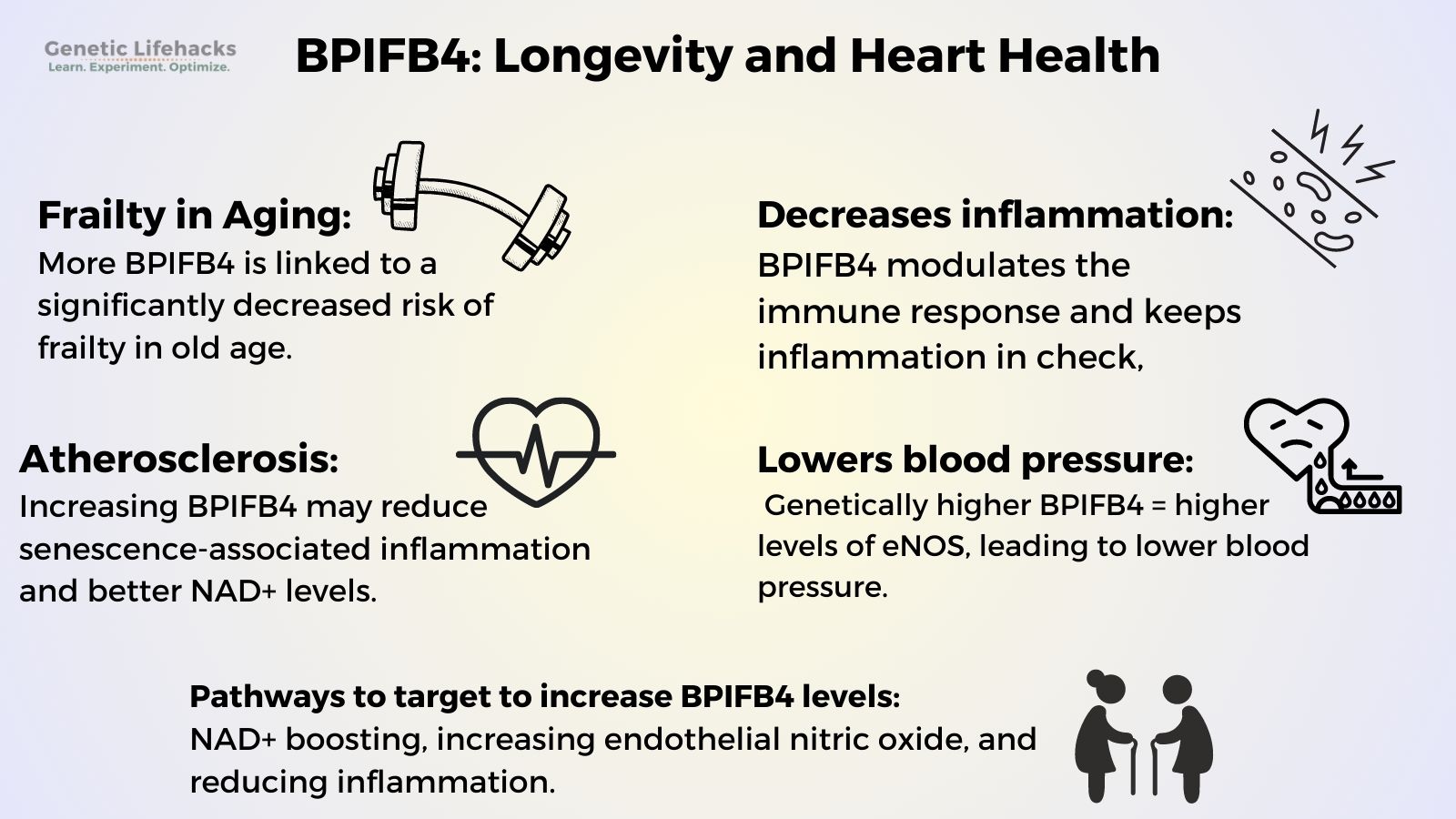
BP1FB4 is involved in promoting more M2 (anti-inflammatory) macrophages.[ref] This keeps the immune response in check and thus prevents elevated inflammation in aging.
Frailty in Aging:
In aging, frailty is marked by decreased stamina, speed, activity, strength, and weight. People who are frail are more likely to have adverse events.[ref]
Gain of function mutations in BPIFB4 are linked to a significantly decreased risk of frailty in old age. Conversely, mutations that decrease the function of BPIFB4 are linked to a significant increase in the risk of frailty.
Atherosclerosis and Heart Disease:
Plaque building up in the arteries causes atherosclerosis, leading to heart disease, high blood pressure, increased heart attack and stroke risk.
A key factor in causing atherosclerosis is chronic, systemic inflammation. Gene therapy studies on BPIFB4 show that it may be able to improve cardiovascular disease. Researchers used adenovirus viral vectors to transfer the longevity variant of BPIFB4 into mice. The animals undergoing the gene therapy had reduced senescence-associated inflammation and better NAD+ levels. Intriguingly, the increased BPIFB4 variant found in humans is also associated with higher NAD+ levels.[ref]
Note that the studies on BPIFB4 gene therapy in animals are financed by a company with a patent on this as a product…
Endothelial nitric oxide synthase:
Nitric oxide is released in the endothelial cells lining blood vessels to cause the blood vessels to relax. Endothelial nitric oxide synthase (eNOS) is the enzyme responsible for creating nitric oxide from arginine. Low levels of eNOS decrease the formation of nitric oxide in the endothelium. This is one cause of higher blood pressure.
Individuals with genetically higher BPIFB4 have higher levels of eNOS, which causes the blood vessels to relax and blood pressure to decrease. Lower blood pressure and more flexibility in blood vessels are linked to better heart health (and thus longevity).[ref]
This may tie back into BPIFB4 increasing the M2 macrophages that modulate the inflammatory response. Chronic inflammation is a driving factor in heart disease and in decreasing eNOS.
Related article: NOS3 gene and endothelial nitric oxide synthase
COVID and BPIFB4
Frailty and old age are the biggest risk factors for mortality in COVID-19.
BPIFB4 plays a role in modulating immune response, and the overactive immune response causes severe Covid symptoms and mortality. Researchers found that circulating levels of BPIFB4 correlated to worse outcomes in Covid. Additionally, the drop in BPIFB4 values correlated to disease severity.[ref]
BPIFB4 Genotype Report:
Lifehacks:
Researchers are looking at ways of using BPIFB4 gene therapy to prevent atherosclerosis and frailty. The monetary potential there seems huge, but we will have to see how well the animal trials translate into humans.[ref]
Without a direct way to increase BPIFB4, here are some options for acting on the same pathways:
The rest of this article is for Genetic Lifehacks members only. Consider joining today to see the rest of this article.
More to come?
Keep in mind that the research on BPIFB4 is pretty new – mostly within the last five years or so. This is one gene that I’m going to keep an eye out for new information.
Graphical Overview:
Related Articles and Topics:
Alpha-Ketoglutarate: A Key to Healthspan and Fighting Age-Related Diseases
Supplementing with Alpha-ketoglutarate (αKG) might be beneficial for longevity since it plays a role in energy production and inflammation reduction.
ADRA1A Receptors: Blood vessel reactions under stress
We have many systems in place to control blood pressure and heart rate. The ADRA1A receptors are part of this system. Discover how variants can influence blood vessel stress response and how others are connected to cognitive changes.
Fighting Chronic Inflammation: Understanding the Role of TNF alpha and Your Genes
Do you feel like you are constantly dealing with inflammation? Joint pain, food sensitivity, etc.? Perhaps you are genetically geared towards a higher inflammatory response. Tumor necrosis factor (TNF) is an inflammatory cytokine that acts as a signaling molecule in our immune system.
Chronic Inflammation: Causes and Natural Solutions
Take a deep dive into the causes of chronic inflammation and learn how to target specific inflammatory pathways to reverse or prevent chronic disease.
References:
Ciaglia, Elena, Francesco Montella, et al. “Circulating BPIFB4 Levels Associate With and Influence the Abundance of Reparative Monocytes and Macrophages in Long Living Individuals.” Frontiers in Immunology, vol. 11, 2020. Frontiers, https://www.frontiersin.org/articles/10.3389/fimmu.2020.01034.
Ciaglia, Elena, Valentina Lopardo, et al. “Transfer of the Longevity-Associated Variant of BPIFB4 Gene Rejuvenates Immune System and Vasculature by a Reduction of CD38+ Macrophages and NAD+ Decline.” Cell Death & Disease, vol. 13, no. 1, Jan. 2022, p. 86. PubMed, https://doi.org/10.1038/s41419-022-04535-z.
Dossena, Marta, et al. “New Insights for BPIFB4 in Cardiovascular Therapy.” International Journal of Molecular Sciences, vol. 21, no. 19, Sept. 2020, p. 7163. PubMed, https://doi.org/10.3390/ijms21197163.
Malavolta, Marco, et al. “LAV-BPIFB4 Associates with Reduced Frailty in Humans and Its Transfer Prevents Frailty Progression in Old Mice.” Aging (Albany NY), vol. 11, no. 16, Aug. 2019, pp. 6555–68. PubMed Central, https://doi.org/10.18632/aging.102209.
Melik, Ziva, et al. “L-Arginine as Dietary Supplement for Improving Microvascular Function.” Clinical Hemorheology and Microcirculation, vol. 65, no. 3, 2017, pp. 205–17. PubMed, https://doi.org/10.3233/CH-16159.
Mortensen, Alan, and Jens Lykkesfeldt. “Does Vitamin C Enhance Nitric Oxide Bioavailability in a Tetrahydrobiopterin-Dependent Manner? In Vitro, in Vivo and Clinical Studies.” Nitric Oxide: Biology and Chemistry, vol. 36, Jan. 2014, pp. 51–57. PubMed, https://doi.org/10.1016/j.niox.2013.12.001.
Puca, Annibale Alessandro, et al. “Single Systemic Transfer of a Human Gene Associated with Exceptional Longevity Halts the Progression of Atherosclerosis and Inflammation in ApoE Knockout Mice through a CXCR4-Mediated Mechanism.” European Heart Journal, vol. 41, no. 26, July 2020, pp. 2487–97. PubMed, https://doi.org/10.1093/eurheartj/ehz459.
Santos-Parker, Jessica R., et al. “Curcumin Supplementation Improves Vascular Endothelial Function in Healthy Middle-Aged and Older Adults by Increasing Nitric Oxide Bioavailability and Reducing Oxidative Stress.” Aging, vol. 9, no. 1, Jan. 2017, pp. 187–208. PubMed, https://doi.org/10.18632/aging.101149.
Villa, Francesco, Albino Carrizzo, Anna Ferrario, et al. “A Model of Evolutionary Selection: The Cardiovascular Protective Function of the Longevity Associated Variant of BPIFB4.” International Journal of Molecular Sciences, vol. 19, no. 10, Oct. 2018, p. 3229. www.mdpi.com, https://doi.org/10.3390/ijms19103229.
Villa, Francesco, Albino Carrizzo, Chiara C. Spinelli, et al. “Genetic Analysis Reveals a Longevity-Associated Protein Modulating Endothelial Function and Angiogenesis.” Circulation Research, vol. 117, no. 4, July 2015, pp. 333–45. DOI.org (Crossref), https://doi.org/10.1161/CIRCRESAHA.117.305875.