With 32 million Americans having food allergies, the prevalence of food allergies has risen 50% in just over a decade. Each year in the US alone, 200,000 people seek medical help for an allergic reaction to a food.[ref]
Food allergies are due to a combination of environmental factors and genetic susceptibility. This article covers the background science of allergies and the genetic variants linked to specific food allergies. It concludes with personalized ‘lifehacks’ for genetics and specific foods.
Food allergies, genetics, epigenetics, and environment
Before we dig into the science of food allergies, let’s make sure we are all on the same page. Often, food intolerance or sensitivity gets mislabeled as being a food allergy. While the outcome of avoiding certain foods is the same, what goes on in the body is completely different for a food allergy vs. a food intolerance.
How are food allergies different than food intolerances?
Food intolerances usually develop from an inability to digest a specific component of food. For example, people who don’t produce the enzyme lactase cannot digest dairy foods that contain lactose. (Check your genes for lactose intolerance) Another example is fructose intolerance, caused by a genetic mutation in the gene that encodes an enzyme needed in the process of breaking down fructose.
Food allergies, on the other hand, are often more severe and involve an immune system response and IgE. The most common food allergens include those found in shellfish, milk, peanuts, tree nuts, eggs, finfish, and wheat.
While the majority of food allergies present during childhood, adults can also develop allergies to foods they have eaten for years.
Nickel allergy can also cross react with foods high in nickel. Read more about genetics and nickel allergy symptoms.
Food sensitivity is a murkier subject. Some testing companies offer IgG food sensitivity tests, which may have some merit for guiding elimination diets, especially for IBS.[ref] There is a lot of debate, though, about the relevance and reliability of these tests.[ref] But that is the topic for another whole article..
In this article, we will be looking at food allergies that involve an immune system response and not food intolerances.
Background science of food allergies:
Food allergies are divided into two categories:
- IgE-mediated food allergies
- Non-IgE mediated food allergies
IgE-mediated food allergies cause the immediate reactions that you think of with allergies – hives, rash, sinus drainage, swelling, shortness of breath, and possibly anaphylaxis. This type of allergy is diagnosed by symptoms along with a skin prick test. The skin prick test inserts the allergen under the skin to see if there is a reaction (redness, swelling).
Non-IgE mediated food allergies can cause either an immediate or a delayed reaction, usually involving the gastrointestinal system. One non-IgE mediated food allergy is Eosinophilic Esophagitis (EoE), in which the esophagus becomes injured and inflamed due to food or environmental allergens.
Oral tolerance – learning not to react to food particles:
Everything you eat breaks down in the gastrointestinal system for absorption – starting with saliva in the mouth, gastric acid, and certain enzymes in the stomach, and then a further enzymatic breakdown in the small intestines.
In the small intestines, your body figures out the difference between the food to absorb versus a pathogen to eliminate. The body constantly surveys and samples for the presence of possible pathogens – or foreign substances for the immune system to eliminate. Dendritic cells in the intestines are at the heart of this system, sorting out nutrients from bacteria.[ref]
In the intestines, proteins found in digested food can pass through the epithelial cells lining the intestines in two ways: passive or active transport. The active transport option includes dendritic cells in the lamina propria, which are sampling proteins to make sure they aren’t ‘bad guys’.[ref]
Also residing in the intestines are a huge array of bacteria, viruses, and archaea – known collectively as your gut microbiome. Thus, food proteins need to be absorbed to nourish your body, and microbes need to be kept out. The immune system has the job of identifying and then destroying any microbes that make it through the intestinal epithelial barrier.[ref]
Dendritic cells are a type of immune cell known as an antigen-presenting cell. They are found in tissues exposed to the outside world, such as the skin, lungs, nose, and intestines. In the intestines, dendritic cells determine whether a particle needs to be presented to T cells and B cells for destruction — and then remembered for future encounters.
Dendritic cells also can present a molecule in a way to show it should be tolerated.
When a normal protein found in food is marked as being a foreign pathogen that needs to be remembered and destroyed, you end up with food allergies.
Adaptive immune response and IgE mediated food allergies:
When a dendritic cell presents a food protein molecule to signal that it is a pathogen, T cells (a type of white blood cell) activate.
Specifically, Th2 cells, which are a subtype of T cell. The Th2 (T helper 2) cells produce interleukin-4 (IL-4) or IL-13, cytokine signals that stimulate B cells to produce IgE (immunoglobulin E).[ref][ref]
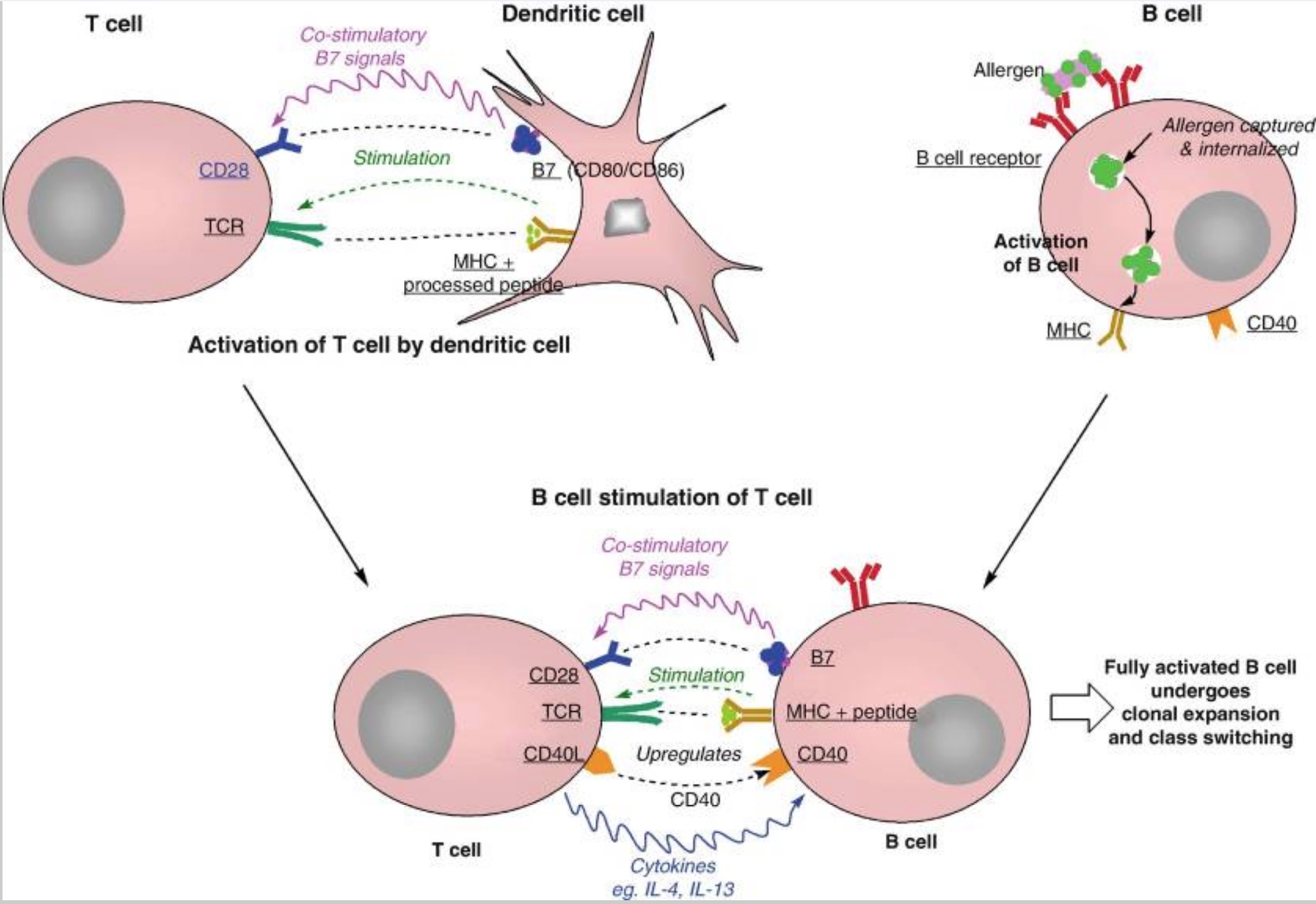
IgE is specific to the food antigen, and when it encounters the same food antigen again, the IgE binds to a receptor on mast cells, triggering degranulation and the release of histamine. Typical allergy symptoms such as hives, itching, and airway constriction result from mast cells releasing a bunch of histamines.
In addition to its role in allergies, IgE is produced to battle helminth (worm) infections, protozoan infections (e.g., malaria), and bee venom.
This gets complicated, so let me recap and explain further:
Th2 cells produce IL-4 and IL-13. These two cytokines induce B cells, another type of immune system cell, to produce IgE against the specific food protein.
IgE then binds to a receptor on mast cells or basophils to release histamine and causes allergic reaction symptoms such as itching, swelling, sinus drainage, or breathing changes.[ref]
When reading about allergies, a lot of focus used to be on the Th2 to Th1 balance. Increased Th2 compared to Th1 cells tips the balance towards allergies, but this is not the complete picture and doesn’t fit with all allergies.
In the early 2000s, Th17 (T helper 17) cells were discovered. Th17 cells are a type of T helper cell important for the immune response in the mucosal barriers, such as intestinal barrier function, and in the lungs and nose. Recent research shows that Th17 (T helper 17) cells are also important in allergy risk.[ref][ref]
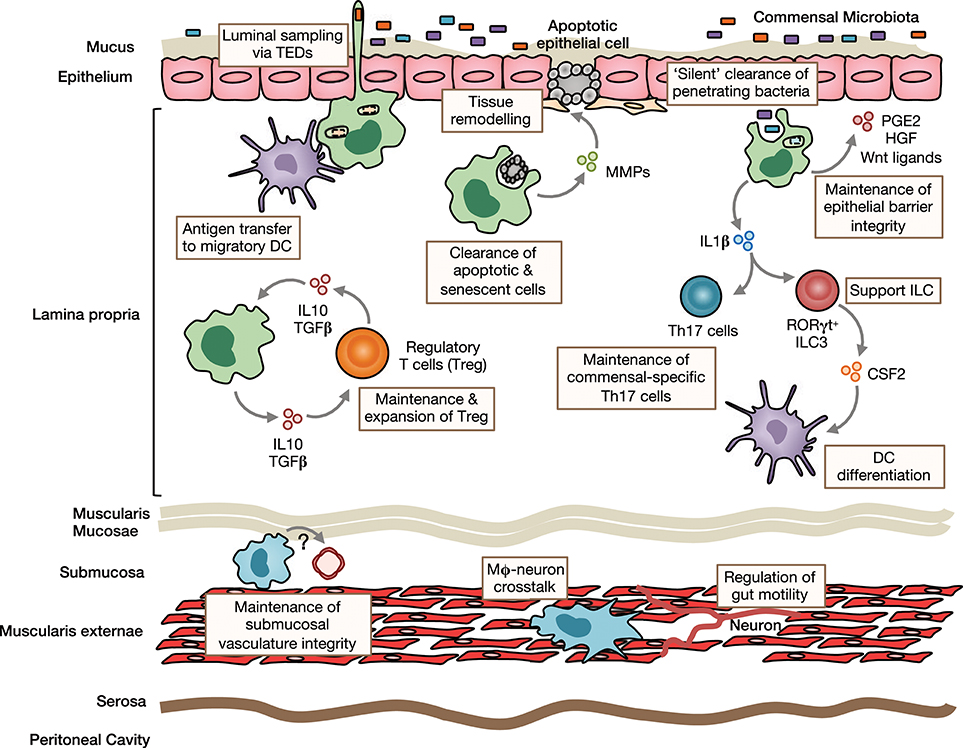
T regulatory cells, keeping it all in check.
The ability of the immune system to ignore food particles (oral tolerance) is an active process in which the immune system remains unresponsive to an antigen. T regulatory cells are a big part of maintaining oral tolerance.
T regulatory (T reg) cells are a specialized subset of T cells that maintain homeostasis – keeping the immune response in balance.[ref] The T regs balance out the Th17 and Th2 responses that can tip the body into an allergic reaction.
A lot of research is going on right now about oral tolerance. Researchers are trying to discover how the T regulatory cells activate and how the activation causes Th2 cells to be dampened or deleted.[ref]
Skin barrier function: Why do eczema and food allergies occur together?
Exposure to allergens through the skin can also be a trigger for food allergies. As you’ll see in the genetics section, there is a strong link between genes that encode skin barrier proteins and food allergies. For example, wheat protein is a common ingredient in skin lotions, which could be a sensitizing factor in some cases.
Atopic dermatitis (eczema) and food allergies go hand in hand. People with atopic dermatitis are much more likely to have food allergies, and eating certain foods can exacerbate atopic dermatitis.[ref] A research study showed that two-thirds of people with eczema have IgE-related reactions to food (allergies or hypersensitization).[ref]
Non-IgE related allergies:
A recent study of 155 patients diagnosed with IBS (irritable bowel syndrome) showed 70% had intestinal tissue reactions to 4 different challenge foods. Of those that reacted to foods, 60% reacted to wheat. All of the patients in the study were negative for IgE-mediated food allergies to the challenge foods. The researchers used confocal laser endomicroscopy along with an intestinal biopsy to examine the changes to the intestinal villi. The results found that the intestinal barrier function was immediately disrupted upon exposure to certain challenge foods. Additionally, increased eosinophil activation was found in the intestines.
Eosinophilic esophagitis (EoE) is another non-IgE allergy condition. In EoE, eosinophils, a type of immune cell, are increased in the esophagus. This leads to increased inflammation, which can make it uncomfortable to eat or swallow. Often misdiagnosed as GERD, it is an allergic inflammation instead. Symptoms of EoE include dysphagia, food impaction, heartburn, abdominal pain, nausea, rhinitis, and hoarseness.[ref]
What’s changed? Why do so many kids now have food allergies?
When reading about food allergies, many researchers refer to it as an ‘epidemic’. The increase in cases over the past couple of decades has been dramatic.
What has changed? That is a hard question to answer because many things have changed in our environment over the past 30+ years.
From antibiotic use to changes in food production, food additives, household chemicals, plastics, childhood vaccination schedules, sunscreen use, and less fiber in the diet — the list of possible reasons for an increase in allergies is long.
This long list of changes makes it easy to come up with a hypothesis that fits a viewpoint…and you will often see on alternative health sites information on allergies to fit an agenda.
Here is one such hypothesis, which may very well be influenced by my own viewpoint:
Folic acid and the rise in food allergies:
A 2020 research study shows how folic acid fortification of foods may impact susceptibility to allergies. The researchers followed ~1400 children from birth through early childhood to see if folate, methyl folate, or unmetabolized folic acid made a difference in developing allergies. The results showed that children within the upper quartile (top 25%) of unmetabolized folic acid levels at birth were at an 8-fold increased relative risk of food allergies. An 8-fold increase in risk is substantial…Additionally, the researchers found that lower folate concentrations at birth were at a higher risk of food allergies.[ref]
This may seem counterintuitive – kids born with lots of unmetabolized folic acid are at a high risk of allergies, but kids with low folate are at a high risk for food allergies. Important here is how the body converts (or doesn’t convert) folic acid into folate.
Folic acid, a synthetic, stable version of vitamin B9, converts in a couple of steps (using a couple of different genes) into the active version of folate, methyl folate (5-MTHF), used by the body in the methylation cycle.
Of interest here is the US-mandated folic acid fortification in the late 90s, with complete implementation in the early 2000s. Folic acid is added to white rice and foods containing wheat flour, such as bread, pasta, and cereals. Additionally, and perhaps more importantly here, prenatal vitamins include large amounts of folic acid. Coincidental with implementing folic acid fortification is the rise in food allergies, with insurance claims for anaphylaxis treatment rising 377% from 2007 to 2016.[ref]
Genetically, some people don’t convert folic acid as quickly, and it can build up in the bloodstream in the unmetabolized form. This unmetabolized folic acid feeds back to inhibit the enzyme that converts folate into an active form. If you are interested in learning more about folic acid metabolism, you can check your folic acid metabolism genes here.
Is folic acid in fortified foods the only possible cause for the increase in food allergies? Of course not. Other explanations include the use of detergents, which have links to infant eczema. Vitamin D deficiency due to sunscreen and sun avoidance has links to increased allergy risk. The ‘Westernized’ dietary pattern is linked to increased food allergies. Low microbial exposure (lack of helminths and other parasites) may also play a role.[ref]
Instead of one single cause applicable to everyone, the genetic variants linked to various food allergies point to multiple reasons for the increase in food allergies.
Genetics, epigenetics, and environmental factors:
To restate from the beginning, allergies come about due to a genetic susceptibility combined with environmental factors.
Genetic susceptibility is important in allergies; the “heritability”, or genetic component, of food allergies is estimated to be 60 – 87%.[ref] In the genetics section below, you’ll find specific variants linked to specific types of foods.
Epigenetics and allergies:
Epigenetics is the turning on and off of genes for translation into their proteins. There are several different ways for cells to control which genes get turned on and off. Epigenetics is at the root of how cells differentiate into different tissues. For example, your heart cells and your skin cells have the same DNA, but they function and look different due to certain genes being turned on or off.
Research shows that people with milk allergies have an epigenetic suppression of IL-10 (anti-inflammatory) and increased production of IL-4 (inflammatory, stimulates Th2).[ref]
Other research shows epigenetic changes reduce Th1 levels in people with food allergies. Reducing Th1 changes the balance of Th2 to Th1 in favor of allergen sensitization.[ref]
Research on asthma also shows that epigenetic changes in interferon-gamma lead to a shift toward Th2 activation.[ref]
Epigenetics ties back to folic acid supplementation as a potential cause of allergies. The methyl groups created from the folate cycle turn on or off genes via the methylation of DNA. In general, increasing folate levels via folic acid supplementation can cause epigenetic changes, as can decreasing folate levels. To get more specific, animal studies show that maternal folic acid supplementation causes epigenetic changes that increase the risk of allergies in offspring.[ref][ref]
Variations in frequency across countries:
Around the world, food allergy prevalence varies quite a bit, which indicates that environmental factors, as well as cultural norms, come into play.
For general food allergies, the prevalence ranges from 15% of kids in the UK to less than 4% in China and Denmark.
It is estimated that about 3% of children in the US are allergic to peanuts, and 9.5% of children in Australia are allergic to eggs. The prevalence of specific food allergies varies quite a bit, depending on the region of the world. For example, peanut allergies are common in the US, UK, and Australia, while wheat allergies are predominant in Thailand and Japan.[ref]
Studies on ethnic groups show that location and environment may be more important than genetic variations amongst population groups. For example, one study explains: “Ethnic Asian children born in Western countries had a 5-fold higher risk of tree nut allergy compared to Asia-born Asian children. In contrast, white children born in Asia had a significantly lower risk of nut allergy than expatriate white children (OR 0.24, 95% CI 0.07–0.74).”[ref]
One difference in regions may be cross-reactivity with pollens or air pollutants found in an area. For example, there is a correlation between areas with high birch pollen and hazelnut allergies. House dust mite allergens have links to an increase in shellfish allergies – in areas with warm, humid climates.[ref]
Food Allergies Genotype Report
Members: Log in to see your data below.
Not a member? Join here.
Why is this section is now only for members? Here’s why…
Lifehacks:
The following is a roundup of the research on allergies, oral tolerance, and associated conditions. Obviously, this is one of those “talk to a doctor” situations, especially for someone with food allergies that cause anaphylaxis. If you have any questions about dealing with allergies, please seek qualified medical advice.
FLG gene variants and the Omega 6 to Omega 3 ratio:
FLG loss of function mutations may lead to increased inflammation with higher arachidonic acid levels. Arachidonic acid is an omega-6 oil found mainly in animal foods, but the body can also create it from linoleic acid (corn oil, soybean oil). While arachidonic acid is an essential fatty acid, having it in the right amount is important. The research here is on the skin, so I don’t know if it would apply to all allergies.[ref]
Our modern diet contains a lot more omega-6 fatty acids than what people ate historically. This is mainly due to the increased consumption of vegetable oils.
Research also shows a higher level of omega-3 fatty acids is linked to a reduced risk of asthma and allergies in kids.[ref]
How can you get more omega-3 fats in your diet? Fish are an excellent source. In addition to adding in fish and seafood, look at decreasing omega-6 fats by decreasing your consumption of fried foods. (Read more about genetics, omega-3s, and fish oil)
Vitamin D and Vitamin A in allergies:
Vitamin D and vitamin A are important for intestinal T regulatory cells. The T reg cells keep the immune response in check, calming down the activated dendritic cells.
Sun exposure and vitamin D: Researchers show that vitamin D deficiency increases sensitization to food allergies in animals, but the studies on supplemental vitamin D in pregnant and breastfeeding women do not show that it prevents food allergies in babies. Thus, this is more nuanced than just ‘take vitamin D’.
Season of birth influences the risk of allergies due to differences in the number of T regulatory cells and other immune cell subtypes. Summer babies had the lowest immune cell types, while winter babies had higher innate immune cells, including activated T cells.[ref] In the northern hemisphere, babies born in the fall or winter are slightly more likely to develop atopic dermatitis and food allergies.[ref][ref]
Mast cells, responsible for histamine release in allergies, are less likely to react in IgE mediated allergies with higher active vitamin D levels. Additionally, higher vitamin D levels help prevent ‘leaky gut’ by increasing the intestinal proteins needed for tight junctions.[ref][ref]
Taken together, the research shows that sufficient vitamin D levels are important, but not the whole story since just taking a vitamin D pill doesn’t seem to prevent food allergies.
Exposure to UV-B (sunshine) increases T regulatory cells – even without vitamin D. The researchers showed this using mice bred to not have vitamin D receptors.[ref]
Related article: Check your vitamin D-related genes
Related Articles and Topics:
Histamine Intolerance & Genetics: Check Your 23andMe Raw Data
When your body has too much histamine, it can cause symptoms collectively known as histamine intolerance. This can be due to excess production of histamine by your body or not being able to break down histamine from foods very well. OR… both!
Are you allergic to grass pollen? It may be genetic. (Member’s Only)
Allergies are usually due to a mix of genetic susceptibility and being exposed to certain triggering molecules. Several different gene variants have ties to an increased risk of grass pollen allergies.
Mast cells: MCAS, genetics, and solutions
Mast Cell Activation Syndrome, or MCAS, is a recently recognized disease involving mast cells that misbehave in various ways. Symptoms of MCAS can include abdominal pain, nausea, itching, flushing, hives, headaches, heart palpitations, anxiety, brain fog, and anaphylaxis. Dive into the research on mast cells, genetics, and solutions.
Top 10 Genes to Check in Your Genetic Raw Data
Let me help to cut through some of the confusion with my personal list of what is important. Below are 10 genes with important variants that can have a big impact on health. Check your genetic data — and read the articles to learn more if you do carry the variant.
Debbie Moon is the founder of Genetic Lifehacks. Fascinated by the connections between genes, diet, and health, her goal is to help you understand how to apply genetics to your diet and lifestyle decisions. Debbie has a BS in engineering from Colorado School of Mines and an MSc in biological sciences from Clemson University. Debbie combines an engineering mindset with a biological systems approach to help you understand how genetic differences impact your optimal health.

