Key Takeaways:
~Sjögren’s syndrome, an autoimmune disorder affecting 1% of the population, attacks specific proteins produced in the body.
~ The first symptoms are usually dry eyes and dry mouth.[ref]
~Genetic variants can increase susceptibility to Sjogren’s — and can point towards natural solutions that may help.
Members will see their genotype report below, plus additional solutions in the Lifehacks section. Join today.
What is Sjögren’s Syndrome?
Sjögren’s syndrome (pronounced SHOW-gren) is a chronic autoimmune disorder that causes inflammation in certain glands. This inflammation leads to various symptoms, including dry eyes and mouth.
Swedish ophthalmologist Henrik Sjögren first identified the condition in 1933.[ref] He noted that patients with this syndrome had “tears and saliva diminished or absent.”
Symptoms of Sjögren’s Syndrome
Sjögren’s syndrome is an autoimmune disease that affects moisture-producing glands. Sjögren’s is a multisystem disorder, and the symptoms and course of the disease can vary quite a bit.
The symptoms of Sjögren’s syndrome can vary from person to person. The most common symptoms include:
- Dry eyes
- Dry mouth
- Dry skin (less common)
According to the Sjogren Foundation, patients may also have:
- Fatigue
- Pain
- Neuropathy
- Hoarse voice
- Cough
- Kidney problems
- Lymphomas
Most Sjogren patients are women diagnosed in mid-life, but the autoimmune disease can also occur in men, children, and the elderly.[ref][ref]
What Causes Sjögren’s Syndrome?
Sjögren’s syndrome is an autoimmune disease that causes the body to mistakenly attack its moisture-producing glands, including the salivary glands, tear ducts, and lacrimal glands.
Saliva is produced in salivary glands; it keeps the mouth moist, aids in swallowing, initiates digestion, and is even important in speech.
In the saliva of people with Sjögren’s, there is an upregulation of proteins involved in innate immunity, wound repair, and cell signaling.[ref][ref] This shows the immune system is attacking healthy cells.
Tears are produced in the lacrimal gland, which is located above the eye towards the outside. Analysis of tears of people with Sjögren’s shows that they have higher levels of TNF-alpha signaling (inflammatory cytokines) and B cell survival.[ref][ref]
Sjögren’s syndrome starts with a triggering event that causes immune system activation. Next, there is dysregulation of the immune response causing the local formation of antibodies that attack healthy cells in the salivary gland and lacrimal gland. For some, these antibodies can also attack other areas of the body.
Here is an excellent visual overview from PMC7408693 (an open access article worth reading).
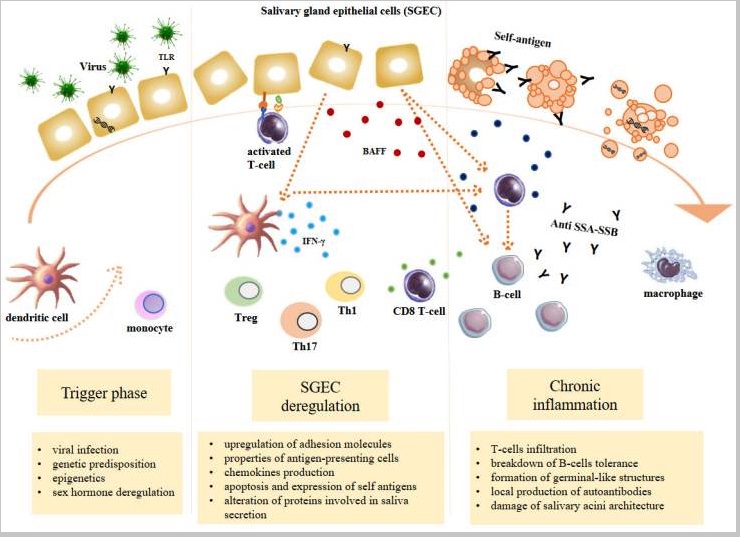
How is Sjögren’s Syndrome Diagnosed?
There doesn’t seem to be yet a consensus on a ‘gold standard’ test to diagnose Sjögren’s syndrome. Some doctors do biopsies of the salivary glands. Most also look at anti-SSA/Ro or anti-SSB antibody tests. Doctors can also use the lack of salivary stimulation or tear stimulation in diagnosing.[ref]
Sjögren’s is defined as primary if it is not associated with other autoimmune diseases. It is considered secondary if it occurs in association with another autoimmune disorder such as lupus, RA, or systemic sclerosis.[ref]
One of the frequent blood tests used for Sjögren’s checks for antibodies to the Ro or La proteins. The antibody test doesn’t definitely show Sjögren’s, though. Some people have anti-Ro/SSA antibodies without having Sjögren’s, so the blood test results are considered along with symptoms and biopsy results.[ref]
Let’s dig into the Ro and La proteins in more depth…
What are anti-SSA/Ro antibodies?
Anti-SSA (also called anti-Ro) is a type of antibody associated with Sjogren’s and other autoimmune diseases (lupus, primary biliary cirrhosis, RA, and systemic sclerosis).
The two different names, SSA or Ro, date back to when the antigens were discovered about five decades ago. SSA stands for Sjögren’s syndrome A. Labs later figured out it was the same antigen, targeting the Ro52 or Ro60 proteins.
So what are these Ro52 and Ro60 proteins targeted by the body’s immune system?
Ro52 is a protein encoded by the TRIM21 gene. It’s a regulatory protein that moderates the inflammatory response.[ref]
Ro60 is an RNA-binding protein found in almost all animals and even in single-celled eukaryotes. Additionally, about 5% of bacteria produce Ro60.[ref] Ro60 acts as a quality checkpoint for RNA, and misfolded RNAs are targeted by Ro60 to be destroyed.[ref]
What are anti-SSB/La antibodies?
About 40% of Sjögren’s syndrome patients have SSB (also called La) antibodies.
A nuclear autoantigen, La/SS-B, is found in both lupus and Sjögren’s patients. It is similar to HMGB1 in that it is an alarmin that can increase immune response.[ref]
New research points to the La protein existing either in an oxidized or reduced form. It refers to whether the protein has an extra electron or not.[ref]
Oxidative stress occurs when there is an excess of reactive oxygen species (ROS) or reactive nitrogen species (RNS) produced by a cell. One reason cells can produce a lot of ROS and RNS is to fight off a pathogen.
Oxidative stress can cause some proteins in a cell to transform (become oxidized) and change the way the protein looks. This change may trigger autoantibodies against proteins that no longer look quite normal. Researchers are also looking into how the oxidized vs. reduced forms of proteins cause rheumatoid arthritis and vitiligo.[ref][ref]
Infections as a trigger of Sjogren’s
Researchers think that infections can be a trigger for Sjögren’s syndrome. The viral reactivation rate of Epstein-Barr virus (EBV) is higher among Sjögren’s patients.
The reactivation of EBV is thought to play a role in the anti-Ro/SSA or the anti-La/SSB antibody production. Coxsackie virus is another possible trigger for Sjögren’s. There is a higher rate of coxsackievirus infection in patients with primary Sjogren’s.[ref][ref]
The Ro60 protein, which is often targeted by antibodies in Sjögren’s, has a peptide in it that is very similar to one in the Epstein-Barr virus. It has led some researchers to suggest EBV triggers the anti-Ro antibodies.[ref]
Gut microbiome – bacteria in the intestines – as a trigger
Sometimes it seems that the gut microbiome is the culprit for all diseases – and all health. I wasn’t expecting the distant gut microbiome to be a possible trigger for autoimmune targeting of the lacrimal and salivary glands, though.
Only a handful of studies specific to the gut microbiome in Sjögren’s syndrome have been done. The few that have been done show less diversity in the microbiome compared to healthy people. For other autoimmune diseases, such as lupus and RA, quite a few studies show altered gut microbiomes in patients.
It’s interesting to note that some gut microbial peptides can activate Ro60, according to animal research. Additionally, some bacteria in the oral microbiome can mimic Ro60.[ref][ref] This may tie the anti-Ro60 antibodies to specific bacteria.
How could the gut microbiome possibly influence such a far away area of the body?
Within the mucosa lining the intestines, there is constant communication between the gut microbiome and the immune system cells.
One thing that is happening is that the bacteria and viruses in the gut are constantly training T cells, which affects the immune system throughout the body. Certain types of T cells, like T regulator cells, keep the immune response in check. Higher production of short-chain fatty acids in the gut, such as from Bacteroides species, pushes the balance towards more T reg cells.[ref]
A key to preventing autoimmunity seems to be having a balance immune system. One that is primed to jump into action when needed but also kept in check so that it doesn’t attack your own cells.
Neuro-Sjogren’s: Neuropathy along with traditional symptoms
Inflammation of the nervous system is a complication of Sjögren’s that some patients experience. Sensory neuropathy is increasingly common in aging with Sjogren’s syndrome. A nerve conduction study in older Sjögren’s patients (avg. age 63) showed that 89% of the patients in the study also had impairment of the sensory nerves.[ref]. Note that this doesn’t mean that 89% of Sjögren’s patients will have neuropathy, but in this study, many of the older Sjögren’s patients had initially presented with nerve issues.
In addition to neuropathy in the peripheral and sensory nerves, in rare cases, Sjogren’s patients may also have spinal cord involvement or even trigeminal nerve involvement.[ref][ref]
Bilirubin as a marker in Sjogren’s
Bilirubin may be best known for causing jaundice in babies who can’t break it down well. It is also known for causing poop to be brown.
So what is bilirubin? It is a pigment produced when heme breaks down in red blood cells. Red blood cells are constantly produced and constantly broken down. The first step in breaking down red blood cells is to strip the heme molecule from the hemoglobin molecule. The heme molecule is then broken down into biliverdin (a green bile pigment) and then further acted on by an enzyme to form bilirubin, which is a yellow-orange pigment.
Beyond its role in the breakdown of heme, bilirubin is easily oxidized to re-form as biliverdin. It essentially means bilirubin acts as an antioxidant — donating an electron as needed to balance out ROS.[ref]
Researchers have discovered that bilirubin levels are, on average, lower in patients with Sjögren’s syndrome. It could indicate that bilirubin plays a protective role as an anti-inflammatory in Sjogren’s. This finding is interesting because bilirubin is easily and commonly measured in blood work.[ref] It also points to the role of antioxidants and oxidative stress in Sjögren’s.
Genetics and Sjögren’s: Pointing to the Cause
Research points to many different genetic variants that increase the relative risk of Sjögren’s. None of these genetic changes cause Sjögren’s on their own. Instead, the variants increase susceptibility when combined with some kind of environmental trigger, such as a viral infection.
Looking at the genetic variants tied to Sjögren’s, though, gives researchers a better idea of the underlying causes of the disease.
The HLA family of genes encodes hundreds of different HLA types. HLA stands for human leukocyte antigen, and these are the cell surface proteins that regulate the immune system. The hundreds of different variants in these HLA genes give rise to humans being able to detect and respond to a vast range of pathogens, including new pathogens that the immune system hasn’t seen before.
The variation, though, in the HLA genes also means that some people are more prone to certain autoimmune diseases than others. For example, people with celiac disease have either HLA-DQ2.5 or HLA-DQ8. Without either of those HLA types, you won’t have celiac disease.
Unsurprisingly, certain HLA types are also linked to a greater risk of Sjögren’s syndrome. Some of these – the ones covered by 23andMe or AncestryDNA data – are listed in the genotype report below.
Many of the other genes that researchers tie to increasing the relative risk of Sjögren’s syndrome include variants in the immune system and inflammatory cytokine genes.
Sjogren’s Syndrome Genotype Report
Members: Log in to see your data below.
Not a member? Join here.
Why is this section is now only for members? Here’s why…
Lifehacks
As always, talk with your doctor or specialist if you have any medical questions, including whether a specific supplement is a good idea for you.
Standard Treatment Recommendations for Sjögrens Syndrome
- Preservative-free eyedrops and lubricating gels are often the first treatment for dry eyes in Sjögren’s. Your doctor or ophthalmologist may also consider other eye drops or eye treatments.[ref]
- Humidifiers to keep air moisture around 40-50% may be helpful in the winter.
- Chewing gum and sipping water may be helpful for dry mouth.
- Xylitol base lozenges may be recommended by the dentist.
- Regularly going to the dentist is recommended. A dry mouth can increase the risk of cavities.
Medications:
Often used as antimalarial drugs, hydroxychloroquine and chloroquine are also used for autoimmune diseases such as lupus, RA, and primary Sjögren’s syndrome. Both drugs are thought to have an immunomodulatory effect.[ref]
One genetic interaction with chloroquine is G6PD deficiency, which you can read more about and check your genes.
Your doctor likely has many other options for medications for Sjögren’s, and there seem to be several drugs in clinical trials right now.
5 Natural Supplements for Sjögren’s that are backed by research
Related Articles and Topics:
HLA B27: Genetic Variant That Increases Susceptibility to Autoimmune Diseases
Our immune system does an awesome job (most of the time) of fighting off pathogenic bacteria and viruses. But to fight off these pathogens, the body must know that they are the bad guys. This is where the HLA system comes in.
PTPN22 and Autoimmune Diseases
The PTPN22 gene plays a pivotal role in that balance, and genetic variants (SNPs) in PTPN22 increase your risk of autoimmune conditions such as vitiligo, alopecia, RA, type 1 diabetes, and autoimmune thyroid diseases.
Chronic Inflammation & Autoimmune Risk: IL17 gene variants
The IL17 gene codes for an important part of the immune system. When it is overactive, IL-17 can contribute to the risk of autoimmune diseases, including rheumatoid arthritis and inflammatory bowel disease.
Small Fiber Neuropathy: Genetics, Causes, and Possible Solutions
Small Fiber Neuropathy (SFN) results in burning pain, numbness, odd sensations, or autonomic nervous system issues. Learn more about the possible causes and potential solutions to this debilitating disorder.
References:
Aqrawi, Lara A., Hilde Kanli Galtung, Beate Vestad, et al. “Identification of Potential Saliva and Tear Biomarkers in Primary Sjögren’s Syndrome, Utilising the Extraction of Extracellular Vesicles and Proteomics Analysis.” Arthritis Research & Therapy, vol. 19, no. 1, Jan. 2017, p. 14. PubMed, https://doi.org/10.1186/s13075-017-1228-x.
Aqrawi, Lara A., Hilde Kanli Galtung, Eduarda M. Guerreiro, et al. “Proteomic and Histopathological Characterisation of Sicca Subjects and Primary Sjögren’s Syndrome Patients Reveals Promising Tear, Saliva and Extracellular Vesicle Disease Biomarkers.” Arthritis Research & Therapy, vol. 21, no. 1, July 2019, p. 181. PubMed, https://doi.org/10.1186/s13075-019-1961-4.
Berndt, Nicole, et al. “Two Be or Not Two Be: The Nuclear Autoantigen La/SS-B Is Able to Form Dimers and Oligomers in a Redox Dependent Manner.” International Journal of Molecular Sciences, vol. 22, no. 7, Mar. 2021, p. 3377. PubMed Central, https://doi.org/10.3390/ijms22073377.
Boccitto, Marco, and Sandra L. Wolin. “Ro60 and Y RNAs: Structure, Functions and Roles in Autoimmunity.” Critical Reviews in Biochemistry and Molecular Biology, vol. 54, no. 2, Apr. 2019, pp. 133–52. PubMed Central, https://doi.org/10.1080/10409238.2019.1608902.
Bolstad, Anne Isine, et al. “Association between Genetic Variants in the Tumour Necrosis Factor/Lymphotoxin α/Lymphotoxin β Locus and Primary Sjogren’s Syndrome in Scandinavian Samples.” Annals of the Rheumatic Diseases, vol. 71, no. 6, June 2012, pp. 981–88. PubMed, https://doi.org/10.1136/annrheumdis-2011-200446.
Butryn, Michaela, et al. “Clinical, Radiological, and Laboratory Features of Spinal Cord Involvement in Primary Sjögren’s Syndrome.” Journal of Clinical Medicine, vol. 9, no. 5, May 2020, p. 1482. PubMed Central, https://doi.org/10.3390/jcm9051482.
Carsons, Steven E., and Bhupendra C. Patel. “Sjogren Syndrome.” StatPearls, StatPearls Publishing, 2022. PubMed, http://www.ncbi.nlm.nih.gov/books/NBK431049/.
Cristina, Cantú-Salinas, et al. “Tumor Necrosis Factor Alpha Promoter-308G/A Polymorphism in Mexican Patients with Patchy Alopecia Areata.” International Journal of Dermatology, vol. 51, no. 5, May 2012, pp. 571–75. PubMed, https://doi.org/10.1111/j.1365-4632.2011.05291.x.
Ding, Cheng, et al. “TNF-α Gene Promoter Polymorphisms Contribute to Periodontitis Susceptibility: Evidence from 46 Studies.” Journal of Clinical Periodontology, vol. 41, no. 8, Aug. 2014, pp. 748–59. PubMed, https://doi.org/10.1111/jcpe.12279.
Dogru, Murat, et al. “Lactoferrin in Sjögren’s Syndrome.” Ophthalmology, vol. 114, no. 12, Dec. 2007, pp. 2366-2367.e4. www.aaojournal.org, https://doi.org/10.1016/j.ophtha.2007.06.027.
dos Reis Neto, Edgard Torres, et al. “Revisiting Hydroxychloroquine and Chloroquine for Patients with Chronic Immunity-Mediated Inflammatory Rheumatic Diseases.” Advances in Rheumatology (London, England), vol. 60, no. 1, 2020, p. 32. PubMed Central, https://doi.org/10.1186/s42358-020-00134-8.
Durães, Cecília, et al. “Polymorphisms in the TNFA and IL6 Genes Represent Risk Factors for Autoimmune Thyroid Disease.” PloS One, vol. 9, no. 8, 2014, p. e105492. PubMed, https://doi.org/10.1371/journal.pone.0105492.
Easley, Justin T., et al. “Aspirin-Triggered Resolvin D1 Versus Dexamethasone in the Treatment of Sjögren’s Syndrome-Like NOD/ShiLtJ Mice – A Pilot Study.” Journal of Rheumatic Diseases and Treatment, vol. 1, no. 4, 2015, p. 027. PubMed, https://doi.org/10.23937/2469-5726/1510027.
Imgenberg-Kreuz, Juliana, et al. “Genetics and Epigenetics in Primary Sjögren’s Syndrome.” Rheumatology (Oxford, England), vol. 60, no. 5, May 2021, pp. 2085–98. PubMed Central, https://doi.org/10.1093/rheumatology/key330.
Jonsson, Roland, et al. “Current Concepts on Sjögren’s Syndrome – Classification Criteria and Biomarkers.” European Journal of Oral Sciences, vol. 126, no. Suppl Suppl 1, Oct. 2018, pp. 37–48. PubMed Central, https://doi.org/10.1111/eos.12536.
Kabeerdoss, Jayakanthan, et al. “In Vitro Effects of Curcumin on Proinflammatory Cytokines and Expression of Their Genes in Minor Salivary Gland Tissue of Patients with Sjogren’s Syndrome.” Rheumatology International, vol. 42, no. 3, Mar. 2022, pp. 545–51. PubMed, https://doi.org/10.1007/s00296-021-04859-7.
Katsiougiannis, Stergios, and David T. W. Wong. “The Proteomics of Saliva in Sjögren’s Syndrome.” Rheumatic Diseases Clinics of North America, vol. 42, no. 3, Aug. 2016, pp. 449–56. PubMed Central, https://doi.org/10.1016/j.rdc.2016.03.004.
Kivity, Shaye, et al. “Infection and Autoimmunity in Sjogren’s Syndrome: A Clinical Study and Comprehensive Review.” Journal of Autoimmunity, vol. 51, June 2014, pp. 17–22. PubMed, https://doi.org/10.1016/j.jaut.2014.02.008.
Langguth, Daman M., et al. “Specific Testing for ‘Isolated’ Anti‐52 KDa SSA/Ro Antibodies during Standard Anti‐extractable Nuclear Antigen Testing Is of Limited Clinical Value.” Journal of Clinical Pathology, vol. 60, no. 6, June 2007, pp. 670–73. PubMed Central, https://doi.org/10.1136/jcp.2006.040360.
Lessard, Christopher J., et al. “Variants at Multiple Loci Implicated in Both Innate and Adaptive Immune Responses Are Associated with Sjögren’s Syndrome.” Nature Genetics, vol. 45, no. 11, Nov. 2013, p. 10.1038/ng.2792. PubMed Central, https://doi.org/10.1038/ng.2792.
Li, Yongzhe, et al. “A Genome-Wide Association Study in Han Chinese Identifies a Susceptibility Locus for Primary Sjögren’s Syndrome at 7q11.23.” Nature Genetics, vol. 45, no. 11, Nov. 2013, pp. 1361–65. PubMed, https://doi.org/10.1038/ng.2779.
Liu, Chenxi, et al. “Association of GTF2I, NFKB1, and TYK2 Regional Polymorphisms With Systemic Sclerosis in a Chinese Han Population.” Frontiers in Immunology, vol. 12, June 2021, p. 640083. PubMed Central, https://doi.org/10.3389/fimmu.2021.640083.
Macri, Angelo, et al. “Evaluation of Oxidative Stress Levels in the Conjunctival Epithelium of Patients with or without Dry Eye, and Dry Eye Patients Treated with Preservative-Free Hyaluronic Acid 0.15 % and Vitamin B12 Eye Drops.” Graefe’s Archive for Clinical and Experimental Ophthalmology = Albrecht Von Graefes Archiv Fur Klinische Und Experimentelle Ophthalmologie, vol. 253, no. 3, Mar. 2015, pp. 425–30. PubMed, https://doi.org/10.1007/s00417-014-2853-6.
Majumder, Poulami, et al. “Association of Tumor Necrosis Factor-α (TNF-α) Gene Promoter Polymorphisms with Aggressive and Chronic Periodontitis in the Eastern Indian Population.” Bioscience Reports, vol. 38, no. 4, Aug. 2018, p. BSR20171212. PubMed, https://doi.org/10.1042/BSR20171212.
Moon, Jayoon, et al. “Can Gut Microbiota Affect Dry Eye Syndrome?” International Journal of Molecular Sciences, vol. 21, no. 22, Nov. 2020, p. E8443. PubMed, https://doi.org/10.3390/ijms21228443.
Nakken, B., et al. “Associations of MHC Class II Alleles in Norwegian Primary Sjögren’s Syndrome Patients: Implications for Development of Autoantibodies to the Ro52 Autoantigen.” Scandinavian Journal of Immunology, vol. 54, no. 4, Oct. 2001, pp. 428–33. PubMed, https://doi.org/10.1046/j.1365-3083.2001.00993.x.
Nezos, Adrianos, et al. “TNFAIP3 F127C Coding Variation in Greek Primary Sjogren’s Syndrome Patients.” Journal of Immunology Research, vol. 2018, 2018, p. 6923213. PubMed, https://doi.org/10.1155/2018/6923213.
Nocturne, Gaetane, et al. “Germline Variation of TNFAIP3 in Primary Sjögren’s Syndrome-Associated Lymphoma.” Annals of the Rheumatic Diseases, vol. 75, no. 4, Apr. 2016, pp. 780–83. PubMed, https://doi.org/10.1136/annrheumdis-2015-207731.
Odusanwo, Olutayo, et al. “Resolvin D1 Prevents TNF-α-Mediated Disruption of Salivary Epithelial Formation.” American Journal of Physiology. Cell Physiology, vol. 302, no. 9, May 2012, pp. C1331-1345. PubMed, https://doi.org/10.1152/ajpcell.00207.2011.
Parisis, Dorian, et al. “Current State of Knowledge on Primary Sjögren’s Syndrome, an Autoimmune Exocrinopathy.” Journal of Clinical Medicine, vol. 9, no. 7, July 2020, p. 2299. PubMed Central, https://doi.org/10.3390/jcm9072299.
Ramadan, Yasmine Kamal. Probiotics as a Prophylaxis to Prevent Clinical Manifestations of Oral Candidosis in Patients With Sjogren’s Syndrome. Clinical trial registration, NCT03840538, clinicaltrials.gov, 13 Feb. 2019. clinicaltrials.gov, https://clinicaltrials.gov/ct2/show/NCT03840538.
Rastmanesh, Reza. “Aquaporin5-Targeted Treatment for Dry Eye Through Bioactive Compounds and Gut Microbiota.” Journal of Ocular Pharmacology and Therapeutics: The Official Journal of the Association for Ocular Pharmacology and Therapeutics, vol. 37, no. 8, Oct. 2021, pp. 464–71. PubMed, https://doi.org/10.1089/jop.2021.0029.
Seeliger, Tabea, Nils K. Prenzler, et al. “Neuro-Sjögren: Peripheral Neuropathy With Limb Weakness in Sjögren’s Syndrome.” Frontiers in Immunology, vol. 10, July 2019, p. 1600. PubMed Central, https://doi.org/10.3389/fimmu.2019.01600.
Seeliger, Tabea, Marten A. Gehlhaar, et al. “Trigeminal Nerve Affection in Patients with Neuro-Sjögren Detected by Corneal Confocal Microscopy.” Journal of Clinical Medicine, vol. 11, no. 15, Aug. 2022, p. 4484. PubMed Central, https://doi.org/10.3390/jcm11154484.
Shamilov, Rambon, and Brian J. Aneskievich. “TNIP1 in Autoimmune Diseases: Regulation of Toll-like Receptor Signaling.” Journal of Immunology Research, vol. 2018, Oct. 2018, p. e3491269. www.hindawi.com, https://doi.org/10.1155/2018/3491269.
Singh, Medha, et al. “Effect of Omega-3 and Vitamin E Supplementation on Dry Mouth in Patients with Sjögren’s Syndrome.” Special Care in Dentistry: Official Publication of the American Association of Hospital Dentists, the Academy of Dentistry for the Handicapped, and the American Society for Geriatric Dentistry, vol. 30, no. 6, Dec. 2010, pp. 225–29. PubMed, https://doi.org/10.1111/j.1754-4505.2010.00158.x.
Sjögren Syndrome: MedlinePlus Genetics. https://medlineplus.gov/genetics/condition/sjogren-syndrome/. Accessed 23 Aug. 2022.
Stappers, M. H. T., et al. “Polymorphisms in Cytokine Genes IL6, TNF, IL10, IL17A and IFNG Influence Susceptibility to Complicated Skin and Skin Structure Infections.” European Journal of Clinical Microbiology & Infectious Diseases: Official Publication of the European Society of Clinical Microbiology, vol. 33, no. 12, Dec. 2014, pp. 2267–74. PubMed, https://doi.org/10.1007/s10096-014-2201-0.
Szymula, Agnieszka, et al. “T Cell Epitope Mimicry between Sjögren’s Syndrome Antigen A (SSA)/Ro60 and Oral, Gut, Skin and Vaginal Bacteria.” Clinical Immunology (Orlando, Fla.), vol. 152, no. 0, 2014, pp. 1–9. PubMed Central, https://doi.org/10.1016/j.clim.2014.02.004.
Tavares, M., et al. “Tumour Necrosis Factor-Alpha (-308G/A) Promoter Polymorphism Is Associated with Ulcerative Colitis in Brazilian Patients.” International Journal of Immunogenetics, vol. 43, no. 6, Dec. 2016, pp. 376–82. PubMed, https://doi.org/10.1111/iji.12289.
Tsigalou, Christina, et al. “Current Insights in Microbiome Shifts in Sjogren’s Syndrome and Possible Therapeutic Interventions.” Frontiers in Immunology, vol. 9, May 2018, p. 1106. PubMed Central, https://doi.org/10.3389/fimmu.2018.01106.
“Understanding Sjögren’s.” Sjögren’s Foundation, https://www.sjogrens.org/understanding-sjogrens. Accessed 23 Aug. 2022.
Utomo, Suyud Warno, and Jemima Fajarin Putri. “Infections as Risk Factor of Sjögren’s Syndrome.” Open Access Rheumatology : Research and Reviews, vol. 12, Nov. 2020, pp. 257–66. PubMed Central, https://doi.org/10.2147/OARRR.S276727.
Walters, M. T., et al. “A Double-Blind, Cross-over, Study of Oral N-Acetylcysteine in Sjögren’s Syndrome.” Scandinavian Journal of Rheumatology. Supplement, vol. 61, 1986, pp. 253–58.
Wang, Yinghan, et al. “Perspectives of New Advances in the Pathogenesis of Vitiligo: From Oxidative Stress to Autoimmunity.” Medical Science Monitor : International Medical Journal of Experimental and Clinical Research, vol. 25, Feb. 2019, pp. 1017–23. PubMed Central, https://doi.org/10.12659/MSM.914898.
Yoshimi, Ryusuke, et al. “Clinical and Pathological Roles of Ro/SSA Autoantibody System.” Clinical and Developmental Immunology, vol. 2012, 2012, p. 606195. PubMed Central, https://doi.org/10.1155/2012/606195.
Zhang, Peng, et al. “Tumor Necrosis Factor-Alpha Gene Polymorphisms and Susceptibility to Ischemic Heart Disease.” Medicine, vol. 96, no. 14, Apr. 2017, p. e6569. PubMed Central, https://doi.org/10.1097/MD.0000000000006569.
Zhang, Zunni, et al. “Clinical Significance of Serum Bilirubin in Primary Sjögren Syndrome Patients.” Journal of Clinical Laboratory Analysis, vol. 34, no. 3, Nov. 2019, p. e23090. PubMed Central, https://doi.org/10.1002/jcla.23090.
Zhao, Jian, et al. “A Missense Variant in NCF1 Is Associated with Susceptibility to Multiple Autoimmune Diseases.” Nature Genetics, vol. 49, no. 3, Mar. 2017, pp. 433–37. PubMed Central, https://doi.org/10.1038/ng.3782.

