Key takeaways:
~ In order to get pregnant, you need healthy egg cells.
~ There are four key ways to improve egg quality, according to research studies.
~ Your genes play a role in how susceptible you are to damage from toxicants or nutrient deficiencies.
~ A multi-pronged approach may be needed to improve egg quality.
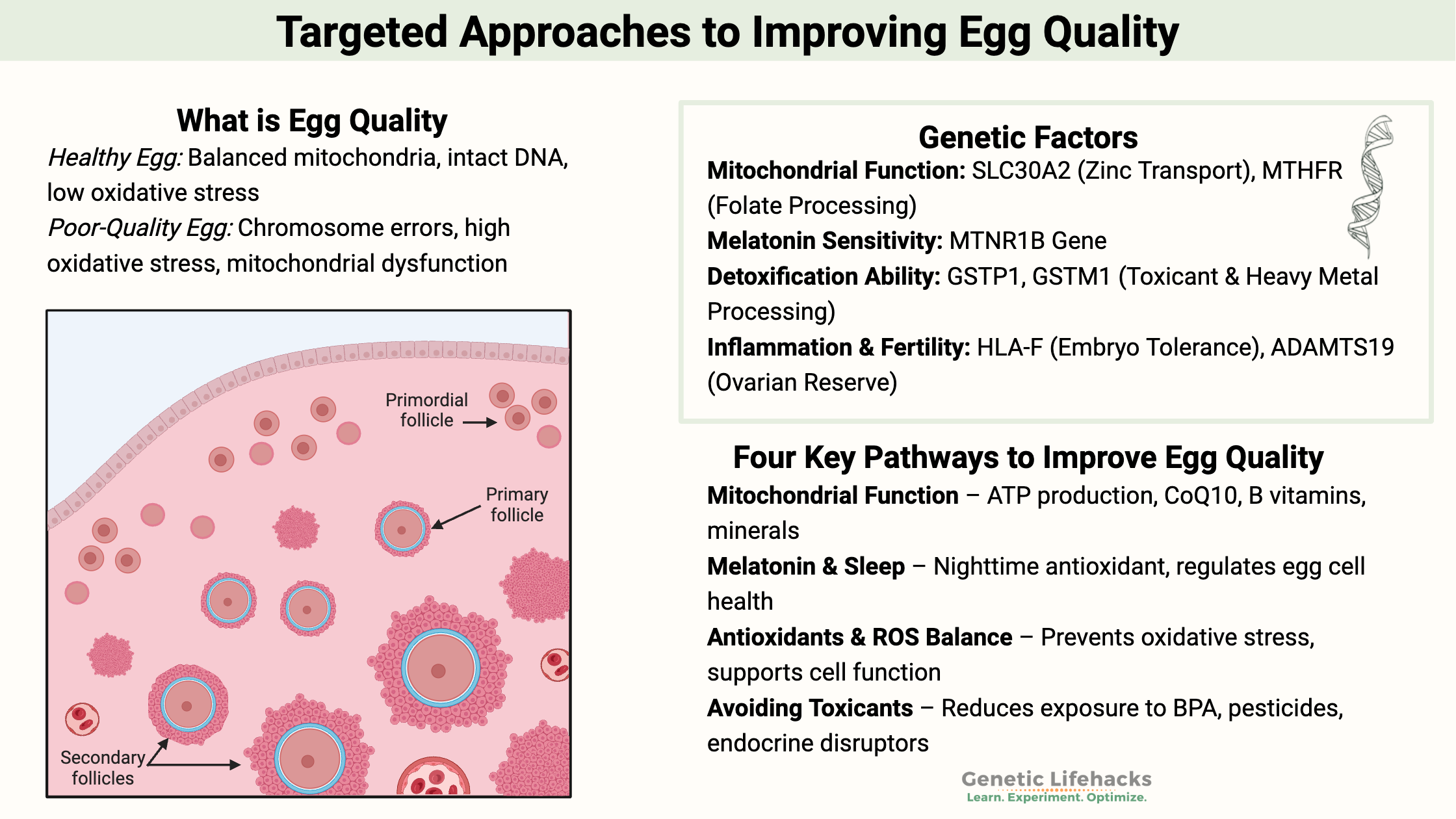
What does ‘egg quality’ even mean?
Many women over 35 who seek help from a fertility specialist are told they have “old eggs” or “poor egg quality.”
What does poor egg quality even mean?
It may surprise you to know that this common phrasing isn’t well-defined in research. But let me explain the gist of what is meant by ‘egg quality’…
All of our cells accumulate damage as we age. It is true for both egg cells and visible cells such as skin and hair. You know the signs of cell damage in aging: gray hair, fine lines, sagging skin, creaking joints…
Similarly, oxidative damage can occur on a smaller scale in the precursor cells for eggs, called oocytes.
One cause of infertility or trouble with conceiving is increased oxidative stress and decreased mitochondrial efficiency in the egg cells. AKA “poor egg quality”.[ref]
Additionally, damage can occur when the DNA replicates as the egg cell matures and prepares for ovulation. Chromosome errors, such as duplications or missing sections, cause the oocyte to be unable to complete development.
Simply put: If the chromosomes are damaged, it usually prevents fertilization of the egg. If the oocyte has mitochondrial dysfunction or excessive oxidative stress, the egg cell cannot develop further. It is the root cause of fertility problems for many women as they age, which is what fertility doctors talk about with ‘egg quality’.
Digging deeper into egg quality:
Every woman, no matter their age, has a percentage of their egg cells that are abnormal or have some damage. Statistically, the percentage of damaged egg cells increases considerably after age 37.[ref]
Fortunately, there is a lot you can do to enhance the overall health of the egg cell.
I’m going to approach egg quality in two ways:
- Solutions for increasing the chances of having an egg with normal DNA
– and – - Solutions for improving the health and quality of the egg cell
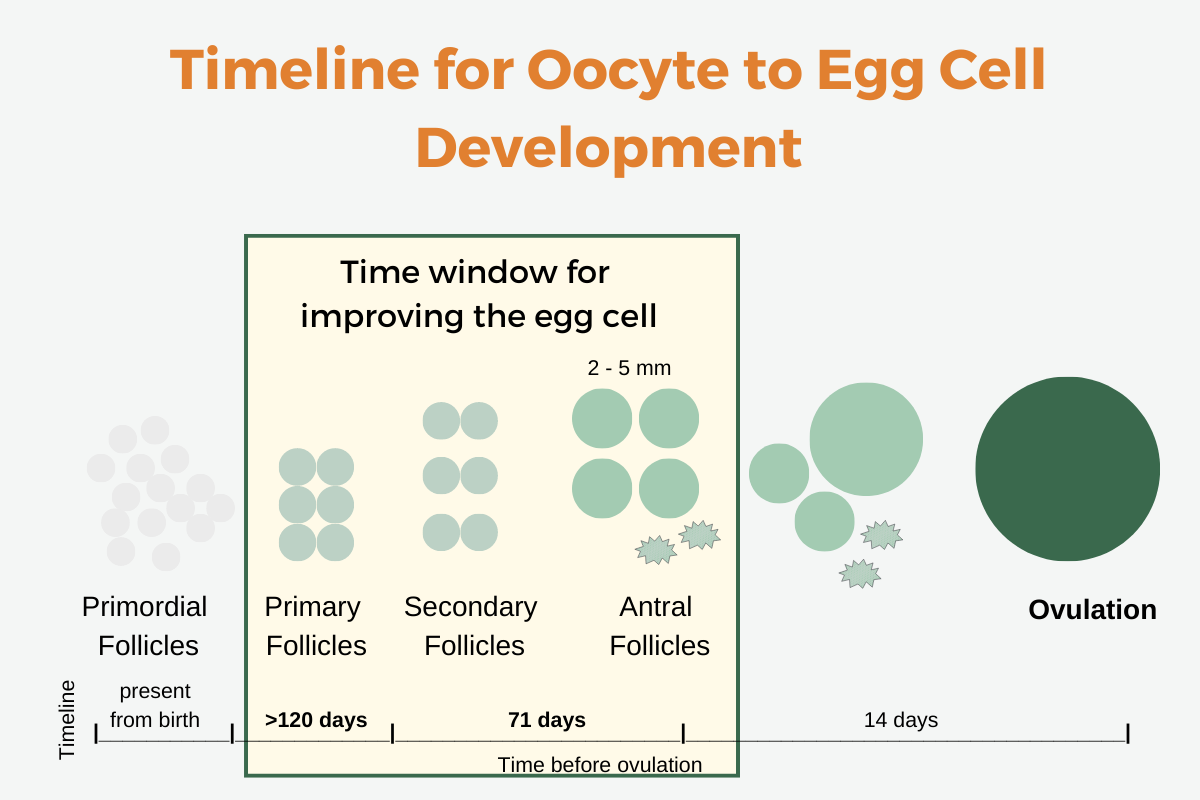
Egg quality improvement doesn’t happen overnight:
Improving egg quality is not a “quick fix”.
Instead, you have to look at improving follicle cells before they develop into the final egg cells for ovulation. Egg quality is not something that can be improved after ovulation. DNA damage must be prevented at the early stages.
The time frame for improving egg quality is two to four months prior to ovulation.
It takes several months for follicles to develop into mature egg cells for ovulation. Follicles contain a single oocyte along with granulosa cells that secrete the sex steroid hormones.
At birth, girls have millions of oocytes in their ovaries. The majority of them will not develop into egg cells for ovulation. However, one will develop into an ovulated egg cell every month (approximately).
Here is a diagram of what happens when primordial follicles prepare to become the ovulated egg cell:
Four pathways to focus on for improving egg quality:
I will focus on four different pathways that can help improve egg cells. These include:
- Improving your mitochondrial function
- Melatonin and quality sleep
- Sufficient antioxidants to balance ROS
- Avoiding toxicants
To maximize the improvement in egg quality, all are important. And each one likely needs to be addressed to some extent. Genetics may help point to where you may want to focus more effort.
First, though, let me explain how these four pathways influence follicles and oocytes.
1) Mitochondrial function:
Mitochondria are the cellular organelles that produce ATP, a molecule used for energy in cellular processes. As oocytes (egg cells) mature, they need a large amount of ATP. Thus, mitochondrial function needs to be optimal.[ref]
Your cells have multiple copies of mitochondria – from hundreds to hundreds of thousands. However, egg cells are at the top of the chart for having the most mitochondria of any cell type, with hundreds of thousands of mitochondria needed.
Fun fact: “A healthy person at rest produces their body weight in adenosine triphosphate every day! At maximal exercise, this number can increase to 0.5 to 1.0 kg per minute —a truly remarkable indication of intense metabolic activity.”[ref]
Mitochondrial dysfunction is a key cause of ovarian aging.[ref]
To have healthy mitochondria, your cells need all the building blocks for producing ATP. It includes vitamins such as riboflavin (vitamin B2) and niacin (vitamin B3), along with glucose or fatty acids for energy.
Minerals and nutrients such as magnesium, iron, manganese, and CoQ10 are also required by your mitochondria.[ref]
In addition to insufficient mitochondrial nutrients, excess reactive oxygen species can cause mitochondrial dysfunction and destruction.
How can this happen?
Many chemicals we are exposed to daily cause oxidative stress, which can impact mitochondrial function.
Here are a few examples:
- Cigarette smoke, alcohol, and radiation exposures are linked to oxidative stress and mitochondrial dysfunction in the egg cell.[ref]
- A recent study showed that aspartame from frequently drinking diet drinks increased the relative risk of infertility in younger women by 79%. The researchers found that in addition to altering anti-Mullerian hormone levels, aspartame also caused mitochondrial stress in the ovaries.[ref]
- Animal studies link BPA, an endocrine disruptor found in plastics, to decreased ovarian function due to decreased mitochondrial membrane potential.[ref]
Related article: BPA, genetics, and detoxification
2) Melatonin:
While we usually think of melatonin as a ‘sleep hormone’, it actually has multiple purposes in cells.
One role of melatonin is as an intracellular antioxidant. In oocytes, melatonin is essential for protecting against oxidative damage.[ref]
Melatonin levels rise dramatically at night as the pineal gland produces a bunch of melatonin. During the daytime, light in the blue wavelengths suppresses melatonin. The changing melatonin levels are one way that your 24-hour circadian rhythm is controlled.
Exposure to bright light during the day increases melatonin production the next night. Additionally, blocking out blue light at night increases melatonin production at the right time. Higher levels of melatonin at night directly act in the oocytes to decrease oxidative stress. Plus, melatonin at the right level and right time increases sleep quality, which is important when trying to conceive.[ref]
Related article: Go in-depth on melatonin
3) Sufficient antioxidants to balance ROS
Balancing out oxidative stress, or the production of reactive oxygen species (ROS), is also very important in the formation of a healthy egg cell.
For the follicle and oocyte to develop into the egg cell that is ovulated, ROS is needed at the right level. Insufficient levels of ROS can inhibit the maturation process, as demonstrated in animal studies. However, with high levels of ROS, the egg cells can be damaged and therefore less likely to result in a pregnancy.[ref]
Some ROS is normal and necessary in a cell. When mitochondria produce energy, a little ROS is also produced. ROS can act as a signaling molecule and facilitates normal cell processes.
Accumulation of ROS in the oocyte can cause cell cycle arrest, halting the normal process of meiosis and preventing the formation of the final egg cell. Additionally, animal studies show that excess ROS in the follicle causes apoptosis or cell death.[ref]
What causes high levels of ROS?
Stress – physiological or psychological stress – can increase ROS and decrease endogenous antioxidant capacity.
4) Avoiding toxicants:
The toxicants you are exposed to on a daily basis – such as BPA, parabens, and phthalates – have been shown to impact fertility to some extent.[ref]
Glyphosate, the herbicide found in RoundUp, has been shown in animal studies to affect fertility a little bit. Human clinical trials are lacking here, and there isn’t a clear picture as to whether glyphosate impacts getting pregnant.[ref]
Organophosphates are another type of pesticide. A recent study in China showed that higher levels of organophosphate exposure were linked to a longer time to conception and a 2-fold increase in the risk of infertility.[ref] Organophosphates are found as residue on some vegetables, as well as in flea and tick products and in home gardening and landscaping.[ref]
Related article: Organophosphates and Genes
Trying to avoid all toxicants, though, is a huge undertaking. Genetic variants related to detoxification may be able to help you determine where to focus your efforts.
Genotype Report: Improving Egg Quality
Lifehacks:
Please talk with your obstetrician or fertility doctor before starting any supplements, especially if you are undergoing fertility treatments.
Aspartame consumption may be detrimental:
Drinking diet drinks that contain aspartame has recently been found to increase infertility risk in younger women (79% increase in relative risk). The study found that aspartame reduced anti-Mullerian hormone (AMH) levels and caused a decline in mitochondrial function.[ref]
Diet and supplements for improving mitochondrial function:
Related Articles and Topics
Folate Optimization: MTHFR and Fertility
MTHFR variants affect the conversion of folate into the active form. Learn how this can affect you if you are trying to get pregnant.
Genetic Causes of Male Infertility
Almost 10% of couples worldwide struggle with infertility. Learn more about your genetic susceptibility and dig deeper into the lifestyle factors that could affect your sperm.
Recurrent miscarriage: Genes and Inflammation
Learn about how genetic variants in certain inflammatory cytokines can increase or decrease the risk of recurrent miscarriage.
Melatonin: Key to Health and Longevity
More than just a sleep hormone, melatonin is at the heart of many health topics. Your genetic variants play a big role in the production of melatonin. Learn how your lifestyle and diet interact with your melatonin-related genes.
References:
Al-Achkar, Walid, et al. “Association of Methylenetetrahydrofolate Reductase C677T and A1298C Gene Polymorphisms With Recurrent Pregnancy Loss in Syrian Women.” Reproductive Sciences (Thousand Oaks, Calif.), vol. 24, no. 9, Sept. 2017, pp. 1275–79. PubMed, https://doi.org/10.1177/1933719116682874.
Barcelos, Gustavo Rafael Mazzaron, et al. “Polymorphisms in Glutathione-Related Genes Modify Mercury Concentrations and Antioxidant Status in Subjects Environmentally Exposed to Methylmercury.” The Science of the Total Environment, vol. 463–464, Oct. 2013, pp. 319–25. PubMed, https://doi.org/10.1016/j.scitotenv.2013.06.029.
Ben-Meir, Assaf, et al. “Coenzyme Q10 Restores Oocyte Mitochondrial Function and Fertility during Reproductive Aging.” Aging Cell, vol. 14, no. 5, Oct. 2015, pp. 887–95. PubMed, https://doi.org/10.1111/acel.12368.
Cain, Sean W., et al. “Evening Home Lighting Adversely Impacts the Circadian System and Sleep.” Scientific Reports, vol. 10, no. 1, Nov. 2020, p. 19110. www.nature.com, https://doi.org/10.1038/s41598-020-75622-4.
Chen, Yang-Ching, et al. “Aspartame Consumption, Mitochondrial Disorder-Induced Impaired Ovarian Function, and Infertility Risk.” International Journal of Molecular Sciences, vol. 23, no. 21, Oct. 2022, p. 12740. PubMed, https://doi.org/10.3390/ijms232112740.
Choi, Youngsok, et al. “Genetic Variation of Methylenetetrahydrofolate Reductase (MTHFR) and Thymidylate Synthase (TS) Genes Is Associated with Idiopathic Recurrent Implantation Failure.” PloS One, vol. 11, no. 8, 2016, p. e0160884. PubMed, https://doi.org/10.1371/journal.pone.0160884.
Custodio, Hipolito M., et al. “Polymorphisms in Glutathione-Related Genes Affect Methylmercury Retention.” Archives of Environmental Health, vol. 59, no. 11, Nov. 2004, pp. 588–95. PubMed, https://doi.org/10.1080/00039890409603438.
de Oliveira, Andréia Ávila Soares, et al. “Genetic Polymorphisms in Glutathione (GSH-) Related Genes Affect the Plasmatic Hg/Whole Blood Hg Partitioning and the Distribution between Inorganic and Methylmercury Levels in Plasma Collected from a Fish-Eating Population.” BioMed Research International, vol. 2014, 2014, p. 940952. PubMed Central, https://doi.org/10.1155/2014/940952.
Dragovic, Sanja, et al. “Effect of Human Glutathione S-Transferase HGSTP1-1 Polymorphism on the Detoxification of Reactive Metabolites of Clozapine, Diclofenac and Acetaminophen.” Toxicology Letters, vol. 224, no. 2, Jan. 2014, pp. 272–81. PubMed, https://doi.org/10.1016/j.toxlet.2013.10.023.
El-Raey, Mohamed, et al. “Evidence of Melatonin Synthesis in the Cumulus Oocyte Complexes and Its Role in Enhancing Oocyte Maturation in Vitro in Cattle.” Molecular Reproduction and Development, vol. 78, no. 4, Apr. 2011, pp. 250–62. PubMed, https://doi.org/10.1002/mrd.21295.
Fabozzi, Gemma, et al. “Personalized Nutrition in the Management of Female Infertility: New Insights on Chronic Low-Grade Inflammation.” Nutrients, vol. 14, no. 9, May 2022, p. 1918. PubMed Central, https://doi.org/10.3390/nu14091918.
Garner, Tyler Bruce, et al. “Role of Zinc in Female Reproduction.” Biology of Reproduction, vol. 104, no. 5, May 2021, p. 976. www.ncbi.nlm.nih.gov, https://doi.org/10.1093/biolre/ioab023.
Gundacker, Claudia, et al. “Genetic Background of Lead and Mercury Metabolism in a Group of Medical Students in Austria.” Environmental Research, vol. 109, no. 6, Aug. 2009, pp. 786–96. PubMed, https://doi.org/10.1016/j.envres.2009.05.003.
Hardy, Madeleine L. M., et al. “Redox Regulation and Oxidative Stress in Mammalian Oocytes and Embryos Developed In Vivo and In Vitro.” International Journal of Environmental Research and Public Health, vol. 18, no. 21, Oct. 2021, p. 11374. PubMed Central, https://doi.org/10.3390/ijerph182111374.
Homer, Hayden Anthony. “The Role of Oocyte Quality in Explaining ‘Unexplained’ Infertility.” Seminars in Reproductive Medicine, vol. 38, no. 1, Jan. 2020, pp. 21–28. PubMed, https://doi.org/10.1055/s-0040-1721377.
Hu, Yi, et al. “Organophosphate and Pyrethroid Pesticide Exposures Measured before Conception and Associations with Time to Pregnancy in Chinese Couples Enrolled in the Shanghai Birth Cohort.” Environmental Health Perspectives, vol. 126, no. 7, July 2018, p. 077001. PubMed Central, https://doi.org/10.1289/EHP2987.
Huang, Po-Chin, et al. “Association between Phthalate Exposure and Glutathione S-Transferase M1 Polymorphism in Adenomyosis, Leiomyoma and Endometriosis.” Human Reproduction (Oxford, England), vol. 25, no. 4, Apr. 2010, pp. 986–94. PubMed, https://doi.org/10.1093/humrep/deq015.
Hubacek, Jaroslav A., et al. “Association of MTHFR Genetic Variants C677T and A1298C on Predisposition to Spontaneous Abortion in Slavonic Population.” Clinica Chimica Acta; International Journal of Clinical Chemistry, vol. 440, Feb. 2015, pp. 104–07. PubMed, https://doi.org/10.1016/j.cca.2014.11.018.
Irimia, Traian, et al. “Oxidative-Stress Related Gene Polymorphism in Endometriosis-Associated Infertility.” Medicina (Kaunas, Lithuania), vol. 58, no. 8, Aug. 2022, p. 1105. PubMed, https://doi.org/10.3390/medicina58081105.
Jahromi, Bahia Namavar, et al. “Effect of Melatonin on the Outcome of Assisted Reproductive Technique Cycles in Women with Diminished Ovarian Reserve: A Double-Blinded Randomized Clinical Trial.” Iranian Journal of Medical Sciences, vol. 42, no. 1, Jan. 2017, pp. 73–78.
Jaramillo-Rangel, G., et al. “Polymorphisms in GSTM1, GSTT1, GSTP1, and GSTM3 Genes and Breast Cancer Risk in Northeastern Mexico.” Genetics and Molecular Research: GMR, vol. 14, no. 2, June 2015, pp. 6465–71. PubMed, https://doi.org/10.4238/2015.June.11.22.
Kirillova, Anastasia, et al. “The Role of Mitochondria in Oocyte Maturation.” Cells, vol. 10, no. 9, Sept. 2021, p. 2484. PubMed Central, https://doi.org/10.3390/cells10092484.
Lee, Sooyeon, et al. “A Genetic Variant in SLC30A2 Causes Breast Dysfunction during Lactation by Inducing ER Stress, Oxidative Stress and Epithelial Barrier Defects.” Scientific Reports, vol. 8, no. 1, Feb. 2018, p. 3542. PubMed, https://doi.org/10.1038/s41598-018-21505-8.
Li, Chao, et al. “Association of Rs10830963 and Rs10830962 SNPs in the Melatonin Receptor (MTNR1B) Gene among Han Chinese Women with Polycystic Ovary Syndrome.” Molecular Human Reproduction, vol. 17, no. 3, Mar. 2011, pp. 193–98. PubMed, https://doi.org/10.1093/molehr/gaq087.
Liu, Yan, et al. “Melatonin Protects against Primary Ovarian Insufficiency by Activating the PI3K/Akt/MTOR Pathway and Inhibiting Autophagy.” Annals of Clinical and Laboratory Science, vol. 52, no. 6, Nov. 2022, pp. 895–903.
Milesi, María Mercedes, et al. “Glyphosate Herbicide: Reproductive Outcomes and Multigenerational Effects.” Frontiers in Endocrinology, vol. 12, July 2021, p. 672532. PubMed Central, https://doi.org/10.3389/fendo.2021.672532.
NM_001004434.3(SLC30A2):C.161A>G (p.His54Arg) AND Zinc Deficiency, Transient Neonatal – ClinVar – NCBI. https://www.ncbi.nlm.nih.gov/clinvar/RCV000032750.3/. Accessed 14 Feb. 2023.
Object, object. Genome-Wide Association Study in Premature Ovarian Failure Patients Suggests ADAMTS19 as a Possible Candidate Gene. core.ac.uk, https://core.ac.uk/reader/148228923?utm_source=linkout. Accessed 14 Feb. 2023.
Oliveira, Tafnes, et al. “Can the Consumption of Ultra-Processed Food Be Associated with Anthropometric Indicators of Obesity and Blood Pressure in Children 7 to 10 Years Old?” Foods, vol. 9, no. 11, Oct. 2020, p. 1567. PubMed Central, https://doi.org/10.3390/foods9111567.
Pan, Meng-Hao, et al. “Bisphenol A Exposure Disrupts Organelle Distribution and Functions During Mouse Oocyte Maturation.” Frontiers in Cell and Developmental Biology, vol. 9, 2021, p. 661155. PubMed, https://doi.org/10.3389/fcell.2021.661155.
Park, Hyo-Jin, et al. “Melatonin Improves Oocyte Maturation and Mitochondrial Functions by Reducing Bisphenol A-Derived Superoxide in Porcine Oocytes In Vitro.” International Journal of Molecular Sciences, vol. 19, no. 11, Oct. 2018, p. 3422. PubMed, https://doi.org/10.3390/ijms19113422.
Pizzorno, Joseph. “Mitochondria—Fundamental to Life and Health.” Integrative Medicine: A Clinician’s Journal, vol. 13, no. 2, Apr. 2014, pp. 8–15. PubMed Central, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4684129/.
Prasad, Shilpa, et al. “Impact of Stress on Oocyte Quality and Reproductive Outcome.” Journal of Biomedical Science, vol. 23, Mar. 2016, p. 36. PubMed Central, https://doi.org/10.1186/s12929-016-0253-4.
Qiu, Juanjuan, et al. “Association between Polymorphisms in Estrogen Metabolism Genes and Breast Cancer Development in Chinese Women.” Medicine, vol. 97, no. 47, Nov. 2018, p. e13337. PubMed Central, https://doi.org/10.1097/MD.0000000000013337.
Shahrokhi, Seyedeh Z., et al. “The Relationship Between the MTHFR C677T Genotypes to Serum Anti-Müllerian Hormone Concentrations and In Vitro Fertilization/Intracytoplasmic Sperm Injection Outcome.” Clinical Laboratory, vol. 63, no. 5, May 2017, pp. 927–34. PubMed, https://doi.org/10.7754/Clin.Lab.2016.161104.
Shi, X., et al. “Maternal Genetic Polymorphisms and Unexplained Recurrent Miscarriage: A Systematic Review and Meta-Analysis.” Clinical Genetics, vol. 91, no. 2, Feb. 2017, pp. 265–84. PubMed, https://doi.org/10.1111/cge.12910.
Snelson, Matthew, et al. “Processed Foods Drive Intestinal Barrier Permeability and Microvascular Diseases.” Science Advances, vol. 7, no. 14, Mar. 2021, p. eabe4841. PubMed Central, https://doi.org/10.1126/sciadv.abe4841.
Spinedi, Eduardo, and Daniel P. Cardinali. “The Polycystic Ovary Syndrome and the Metabolic Syndrome: A Possible Chronobiotic-Cytoprotective Adjuvant Therapy.” International Journal of Endocrinology, vol. 2018, July 2018, p. e1349868. www.hindawi.com, https://doi.org/10.1155/2018/1349868.
Srour, Bernard, et al. “Ultra-Processed Food Intake and Risk of Cardiovascular Disease: Prospective Cohort Study (NutriNet-Santé).” The BMJ, vol. 365, May 2019, p. l1451. PubMed Central, https://doi.org/10.1136/bmj.l1451.
Thaler, C. J. “Folate Metabolism and Human Reproduction.” Geburtshilfe Und Frauenheilkunde, vol. 74, no. 9, Sept. 2014, pp. 845–51. PubMed Central, https://doi.org/10.1055/s-0034-1383058.
Tomza-Marciniak, Agnieszka, et al. “Effect of Bisphenol A on Reproductive Processes: A Review of in Vitro, in Vivo and Epidemiological Studies.” Journal of Applied Toxicology: JAT, vol. 38, no. 1, Jan. 2018, pp. 51–80. PubMed, https://doi.org/10.1002/jat.3480.
Wang, I.-Jen, and Wilfried J. J. Karmaus. “Oxidative Stress-Related Genetic Variants May Modify Associations of Phthalate Exposures with Asthma.” International Journal of Environmental Research and Public Health, vol. 14, no. 2, Feb. 2017, p. 162. PubMed, https://doi.org/10.3390/ijerph14020162.
Yang, Jae Jeong, et al. “Estrogen Receptor-1 Genetic Polymorphisms for the Risk of Premature Ovarian Failure and Early Menopause.” Journal of Women’s Health (2002), vol. 19, no. 2, Feb. 2010, pp. 297–304. PubMed, https://doi.org/10.1089/jwh.2008.1317.
Zeng, Shuangshuang, et al. “MTHFR C677T Polymorphism Is Associated with Follicle-Stimulating Hormone Levels and Controlled Ovarian Hyperstimulation Response: A Retrospective Study from the Clinical Database.” Fertility and Sterility, vol. 111, no. 5, May 2019, pp. 982-990.e2. ScienceDirect, https://doi.org/10.1016/j.fertnstert.2019.01.016.
Zhang, Mianqun, et al. “Insufficiency of Melatonin in Follicular Fluid Is a Reversible Cause for Advanced Maternal Age-Related Aneuploidy in Oocytes.” Redox Biology, vol. 28, Jan. 2020, p. 101327. PubMed, https://doi.org/10.1016/j.redox.2019.101327.
Zhang, Yixiang, et al. “Association between GSTP1 Ile105Val Polymorphism and Urinary System Cancer Risk: Evidence from 51 Studies.” OncoTargets and Therapy, vol. 9, 2016, pp. 3565–69. PubMed, https://doi.org/10.2147/OTT.S106527.
Zhang, Yonglan, et al. “Glutathione S-Transferase Gene Polymorphisms and Risk of Nasal or Colorectal Polyposis.” Bioscience Reports, vol. 39, no. 1, Jan. 2019, p. BSR20181226. PubMed, https://doi.org/10.1042/BSR20181226.