Key takeaways:
- GLP-1 is a rapid-acting gut hormone that regulates blood sugar and appetite by signaling insulin release and suppressing glucagon.
- GLP-1 controls specific types of appetite rather than baseline hunger. Acute stress and large meals trigger GLP-1 in the brain to suppress appetite, preventing overeating.
- GLP-1 receptor agonist drugs (like Ozempic and Wegovy) work by mimicking natural GLP-1 but last much longer.
- Genetic variants in the GLP1-related genes are linked to obesity and diabetes. Variants influence whether someone is likely to respond well to GLP-1 RAs for weight loss.
- Beyond weight loss, GLP-1 RAs show promise for cardiovascular disease, dementia prevention, and immune conditions. Side effects are common and of concern, though.
Glucagon-like Peptide-1(GLP-1) Hormone Signaling: How it’s produced and what it does
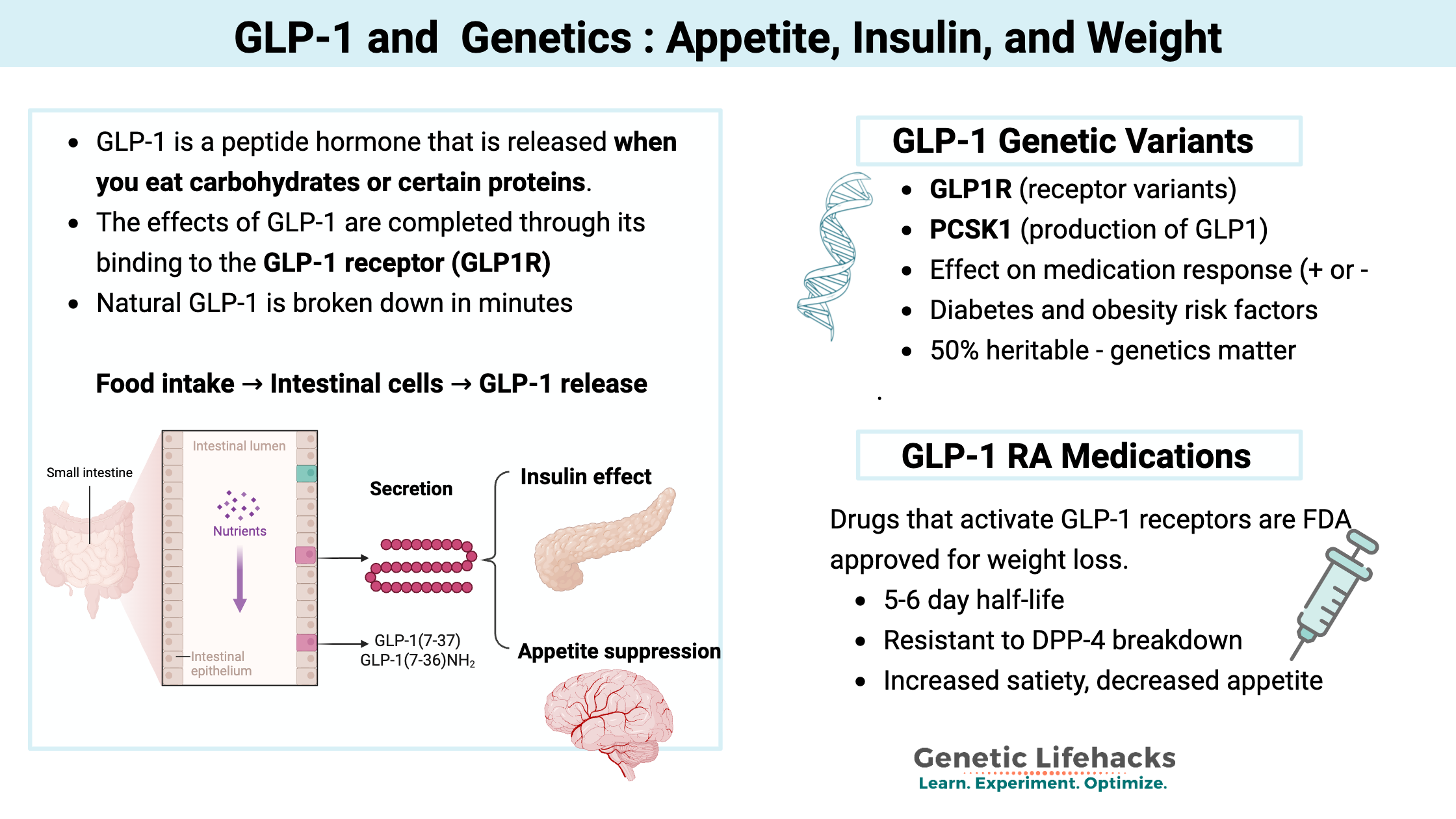
When food reaches your intestine, it triggers the release of hormones that signal to other parts of your body. These signals tell your brain that you’re no longer hungry and prompt the pancreas to release insulin.
One of these hormones is GLP-1 (glucagon-like peptide-1).
Gut-brain axis:
GLP-1 is a peptide hormone (a small protein molecule) that is released in the upper part of the intestinal tract when you eat carbohydrates or certain proteins. It is classified as an incretin, which means it is a hormone released to regulate blood glucose levels.
GLP-1 receptors (GLP1R), which are found in pancreatic beta cells as well as other cell types, receive the GLP-1 signal, triggering the release of insulin. GLP-1 receptors are also found in other cells in the pancreas, where the signal then stops glucagon, which prevents the liver from generating glucose.[ref] Additionally, GLP-1 is created in the brain and acts as a neuropeptide.
To understand how GLP-1 works, we first need to look at how the body produces this hormone.
How is GLP-1 synthesized?
The production of GLP-1 begins with a larger precursor molecule.
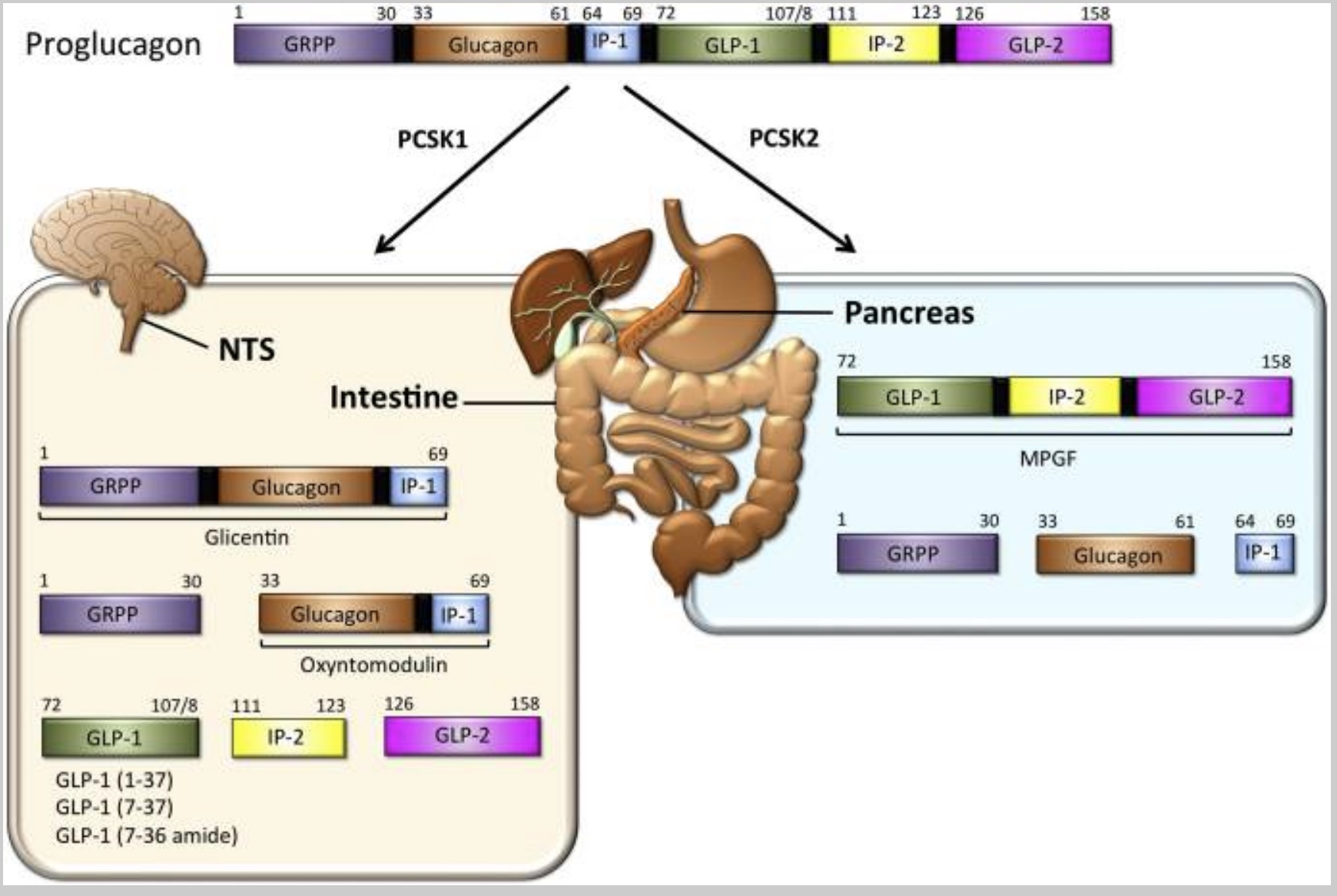
Proglucagon is a large precursor hormone molecule produced in the pancreas (alpha cells) and the intestines. It can be chopped apart to form other molecules — including GLP-1. Preproglucagon is encoded by the GCG gene and cleaved into proglucagon.
Beyond appetite regulation, GLP-1 plays a critical role in blood sugar management.
Controlling glucose levels:
In the pancreas, the proglucagon can convert into glucagon, which in turn signals the liver to release glucose. The alpha cells in the pancreas secrete proglucagon when blood glucose levels are low. The release of proglucagon causes the liver to make glucose (gluconeogenesis – converting protein to glucose) as well as to convert fatty acids into ketone bodies.
Intestinal proglucagon cleaves (breaks up) into several different molecules:
- GLP-1
- GLP-2
- GRPP
- Oxyntomodulin
The enzyme PCSK1 converts proglucagon to GLP-1 in the central nervous system. (We’ll come back to this in the genetics section)
The following diagram illustrates how proglucagon is cleaved into its various components:
GLP-1 signal to the pancreas: Quick signal, rapidly degraded
The GLP-1 hormone has a remarkably short half-life of around 2 minutes. This quick signal is rapidly degraded by the protein DPP-4 (dipeptidyl peptidase 4).
As a result of this rapid degradation, only about 10-15% of released GLP-1 reaches the pancreas intact. Blocking the degradation of GLP-1 by inhibiting DPP-4 is how certain types of diabetes medicine work.
The GLP-1 that does reach the pancreas calls for the release of more insulin when food is being broken down and absorbed. Plus, it stops the production of glucagon, thus decreasing glucose from the liver.
The effects of GLP-1 are mediated through its binding to the GLP-1 receptor (GLP1R).
GLP-1 constant, low-level production and the role of the gut microbiome:
The focus with weight loss and diabetes is the spike in GLP-1 after eating that is quickly broken down. This spike is the signal to the pancreas for insulin release and the full signal to the brain.
However, we also have a constant, low level of circulating GLP-1 even when fasting.[ref][ref] This directly ties into the gut microbiome. A healthy gut microbiome with diversity produces short-chain fatty acids, causing GLP-1 release. Bile acids can also stimulate GLP-1.[ref]
Studies using germ-free mice show that GLP-1 secretion after a meal is abolished without the gut microbiome.[ref]
Are GLP-1 levels genetic?
The heritability of GLP-1 release is around 50%.[ref] Thus, while there is a fairly important genetic component, diet is also really important.
Common genetic variants in the GLP1R (GLP-1 receptor) gene impact weight and type 2 diabetes risk. Variants in the DPP4 gene also affect GLP1 levels and diabetes risk. These are covered in detail in the genotype report section below.
GLP-1 and appetite suppression:
GLP-1 regulates appetite through several distinct mechanisms that affect the brain and the gut.
GLP-1’s role in the brain:
Different regions in the brain control your desire to eat, and GLP-1 comes into play here in specific ways. The hypothalamus can make GLP-1, as well as regions of the brain stem. Plus, GLP-1 is involved in stress response and the HPA axis.[ref]
The brain stem controls a lot of our basic, involuntary functions, such as heart rate and breathing. Specific neurons in the brain stem control swallowing, intestinal movements — and stress-induced loss of appetite.
GLP-1 in the brain stem is activated due to stress and causes a loss of appetite. Researchers determined this using transgenic mice, allowing them to turn off the GLP-1 in the brain to see what happens. The researchers found that turning off GLP-1 in the brain didn’t affect the normal daily intake of food, but instead was important in several specific situations: prolonged fasting, large feedings, and stress-induced appetite loss.[ref]
- Controlling binging, regulating satiety:
Essentially, the neurons activated by GLP-1 help to limit binging after a fast – they keep you from massively overeating after not eating for a while. The GLP-1-activated neurons in the brain also limit appetite for a while after a large meal (e.g., the thought of eating is unappealing for many hours after pigging out on pizza).[ref] - Stress-related appetite suppression:
Additionally, the GLP-1 neurons activate during times of acute stress to suppress appetite. We all have experienced that loss of appetite during stressful situations – when the thought of eating food just doesn’t cross the mind.
Gut-brain axis:
The GLP-1 produced in the intestines after a meal also plays a role in suppressing eating, likely through different circuits than those GLP-1-expressing neurons found in the brain stem. Researchers think that this involves the vagus nerve.[ref]
GLP-1 receptor agonist drugs:
Given GLP-1’s powerful effects on appetite and glucose regulation, researchers developed synthetic medications that mimic its action.
The receptor for GLP-1 is the GLP-1 receptor (GLP1R gene, below in the genotype report section), a G-protein-coupled receptor. The GLP-1 receptor is a type of cell surface receptor that binds to GLP-1, triggering a signaling cascade that leads to various effects, including the stimulation of insulin secretion, the inhibition of gastric emptying, and the promotion of satiety.
History: In the early 2000s, GLP-1-based drugs (exenatide and liraglutide) were developed for the treatment of type 2 diabetes. Semaglutide was approved in 2017 and sold under the brand name Ozempic.
These drugs are GLP-1 receptor agonists. An agonist is a type of drug or other agent that binds to a receptor on a cell and triggers a physiological response.
More recently, GLP-1 RA drugs have been approved for weight loss, cardiovascular disease, and chronic kidney disease. The following GLP-1 (glucagon-like peptide-1) receptor agonists are FDA-approved for weight loss:[ref]
- Semaglutide – GLP-1 receptor agonist (Ozympic, Wegovy)
- Liraglutide – GLP-1 receptor agonist (Saxenda)
- Tirzepatide – GLP1/GIP (Mounjaro, Zepbound)
Retatrutide is a GLP-1/GIP/GCG triple agonist that is in trials for weight loss and diabetes (2025).
Understanding the mechanism behind these medications helps explain their effectiveness.
How does a GLP-1 receptor agonist work for weight loss?
By binding to the GLP-1 receptor, GLP-1 RAs mimic the effects of having eaten and released GLP-1. In other words, you’ll feel full and won’t be driven to eat as much. The key is that GLP-1 RA medications are long-lasting receptor agonists that have an effect lasting much longer than naturally produced GLP-1. The medications resist being degraded by DPP-4, which is the enzyme that breaks down naturally produced GLP-1 within a few minutes. Semaglutide, when used as a subcutaneous injection, has a half-life of 5-6 days. Oral formulations have a shorter half-life.[ref]
In clinical trials, GLP-1 RAs improved lipid profiles, increased satiety, slowed gastric emptying, reduced inflammation, and had neuroprotective effects.[ref] The slowing of gastric emptying and reducing inflammation may play a role in weight loss, along with the decrease in appetite and eating less.
A combined effect that is bigger than just decreasing calories is thought to be at play when looking at the effects of activating the GLP-1 receptor.[ref]
In overweight adults aged 45+, a 4-year-long study using semaglutide for weight loss also prevented HbA1c from rising over time, which was seen in the placebo arm. The conclusion was that semaglutide for weight loss helps prevent overweight adults from progressing to diabetes.[ref]
Beyond metabolic effects, recent research has revealed GLP-1’s broader impact on the immune system.
A 2026 study in mice also ties GLP1 receptor agonist effectiveness for weight loss to the body’s ability to use fatty acids for energy. Carnitine plays an essential role in the way that mitochondria use fats for fuel, and the study showed that mice deficient in a carnitine transporter, SLC25A45, didn’t lose weight as easily on a GLP-1 RA medication.[ref]
Related article: Carnitine genes
GLP-1 and immune response:
In addition to their role in pancreatic insulin secretion, GLP-1 receptors are found in certain types of immune cells, including T cells and macrophages.[ref] This is an interesting link between metabolic health and immune health, and microdosing GLP-1 medications is being looked at for preventing immune dysregulation in aging.
- Immune system modulation:
The receptor for GLP-1 is found on the cell membrane of macrophages, regulatory T cells, and natural killer T cells. GLP-1 plays an immunomodulatory role. A GLP-1 receptor agonist medication may help patients with psoriasis.[ref]
Related article: T Cell Exhaustion
- Mast cell stabilizer:
GLP-1 receptors are also found on mast cells, and recent studies have shown that GLP-1 RA drugs prevent overactive mast cell degranulation. This may be of benefit for anyone with MCAS, and clinical trials are looking at the effectiveness of different doses.[ref][ref] (Related article: MCAS) - Asthma symptoms decrease:
Asthma exacerbations also decreased in patients on GLP-1 receptor agonist medications.[ref] (Related article: Asthma genes)
GLP-1 RAs, dementia, and Alzheimer’s disease prevention:
In animal studies, infusions of GLP-1 decrease amyloid-beta levels and prevent neuronal cell death.[ref]
Dysregulation of glucose entry into the brain is one hallmark of Alzheimer’s disease, tying together insulin resistance, GLP-1, and dementia.[ref][ref] GLP-1 receptor agonist drugs, such as liraglutide, are being studied for Alzheimer’s disease.[ref]
Studies using data from diabetes patients showed a significant decrease in dementia for those on GLP-1s vs other diabetes medications. Some studies showed prevention benefits even in the absence of metabolic changes.[ref][ref]
Related article: APOE genotype and Alzheimer’s risk
Cardiovascular disease benefits:
A number of studies have shown that GLP-1 RAs provide benefits for reducing cardiovascular disease. The medications lower lipid levels and lower blood pressure in both diabetic and weight loss patients. Interestingly, the mechanism may be more than just weight loss. GLP-1 receptors are expressed in the heart muscle cells, immune system cells in the heart, and in the lining of blood vessels.[ref]
Related article: Cardiovascular disease genetic risk factors
Cancer treatment?
Retatrutide, a GLP-1/GIP/GCG triple agonist, has recently been shown to reduce pancreatic tumor mass in animals. First, let me caution that this is just a mouse study, so it may not hold true in humans. A prior study had shown that retatrutide and semaglutide reduced pancreatic tumor mass in mice, along with weight loss. But the interesting thing about the new study is that the researchers used a low dose of retatrutide – one that was too low to cause weight loss. They found that even without causing weight loss, retatrutide had anti-tumor effects that were similar to the currently used (and incredibly expensive) immunotherapy.[ref]
Semaglutide has also been shown to have immunomodulatory and antitumor effects in a mouse model of breast cancer. In this study, the semaglutide promoted T cells to be able to respond more effectively to cancer. [ref]
More research is needed here, of course. Initial studies in rats caused concerns over GLP-1 RAs causing thyroid cancer, but human studies are less clear on whether it is a concern.[ref]
Vagus nerve interaction:
A 2026 study showed that the GLP-1 receptor is expressed in the nodose neurons, which are the sensory cells that transmit information from the internal organs to the brainstem.[ref]
Role of Circadian Rhythm in GLP-1:
The effectiveness of GLP-1 is also influenced by our body’s natural daily rhythms.
Your circadian rhythm is the 24-hour rhythm of your body’s functions. It controls when hormones are released, the production of enzymes at different times of the day, immune response during the day vs. night, sleep-wake timing, and much more.
Circadian control of response to GLP-1:
Like many hormones, there is a circadian aspect to GLP-1. Research points to GLP-1 as having a role in entraining the circadian rhythm of insulin release in the pancreas. While GLP-1 will be released from intestinal cells upon consumption of food, the amount of insulin release that is triggered by GLP-1 varies due to the time of day.[ref]
Diet changes rhythm:
A ‘Western Diet’, high in fat and sugar, is used in research studies to induce obesity in animal models. Studies show that this type of diet actually alters the circadian rhythm of GLP-1 secretion, both in lab animals and in people with type 2 diabetes.[ref]
Does a decrease in natural GLP-1 secretion explain the obesity epidemic?
If you’re like me, you may be wondering what caused obesity to skyrocket over the past three decades – and whether GLP-1 has a role.
Often, the rise in obesity is explained as being due to people eating more and food being more appealing. However, that doesn’t quite fit with the availability of food over the century, the abundance of tasty cakes and pies, and the prior consumption of sugar (which has fallen since the 90s) and butter.
GLP-1 medications are known for quieting the ‘food noise’, meaning that they reduce the constant thoughts about eating, along with increasing satiety. At low doses, they take people back to a more normal food consumption pattern.
GLP-1 is produced in the cells lining the intestines, which makes gut health important. Multiple studies have tied changes in the gut microbiome to lower GLP-1 levels, but the mechanism isn’t always clearly defined.
A 2025 study in mice showed that prenatal glyphosate exposure at normal levels reshapes the gut microbiome. (Glyphosate is the herbicide in Roundup, and the use of glyphosate has risen dramatically over the last three decades. )
Specifically, the changes to the gut microbiome composition caused a reduction in the intestinal cells that produce mucin, which is the barrier that prevents microbes from causing inflammation in the gut. The changes resulted in a decrease in serum GLP-1 along with insulin resistance and metabolic dysfunction. The researchers suggest that changes in gut microbiome composition, particularly involving Akkermansia muciniphila and Parabacteroides distasonis, may explain the effects on GLP-1 and other gut-brain signaling molecules. Other studies have also linked glyphosate to metabolic dysfunction and changes to the gut microbiome, but this was the first study to also look at GLP-1 levels.[ref] [ref]
Related article: Glyphosate, genetics, metabolism
Another ubiquitous environmental exposure is BPA and BPS, which are chemicals used in plastics that act as endocrine disruptors. BPA and BPS exposure have been tied in numerous studies to metabolic dysfunction and obesity. It gets a little complicated, but essentially, BPA/BPS cause changes in a specific microRNA that controls GLP-1 levels. Cell studies show that BPA exposure directly causes a decrease in GLP-1 and insulin release through miR-338.[ref][ref]
Related article: BPA, BPS, Genetics, and Detoxification
Side effects of GLP-1 RA medications:
While GLP-1 RAs offer significant benefits, it’s important to understand their potential drawbacks. They aren’t right for everyone, and the side effects can be significant.
Please talk with your doctor about the risks and side effects before starting any GLP-1 medication.
Gastrointestinal adverse events:
The most common adverse effect is ‘gastrointestinal adverse events’. For example, in a semaglutide clinical trial in teens, gastrointestinal adverse events were reported in 62% of the group taking semaglutide, compared to 42% in the placebo group. Nausea, diarrhea, vomiting, and constipation are all gastrointestinal adverse events, and most occur within the first month of starting.[ref][ref]
Note that for many people, gastrointestinal side effects seem to be dose-related. Microdosing or starting with a lower dose may help to prevent gastrointestinal side effects. Talk with your doctor about the balance between weight loss (if that is your goal) and lowering the dose.
Pancreatitis:
Case studies and a few of the clinical trials on GLP-1 RAs have raised the possibility of pancreatitis being triggered by these drugs due to the potential growth in cells in the pancreas. As an example, one case study involving someone with a history of alcoholic pancreatitis tied an acute pancreatitis episode to starting semaglutide three months before. The FDA Adverse Event Reporting System shows a likely link between GLP-1 RAs (specifically liraglutide) and increased risk of pancreatitis. Clinical trial data also show that pancreatitis is a possible side effect (5 out of 1879 diabetes patients). However, in a large study involving people with diabetes who had no comorbidities, GLP-1 RAs were not shown to increase the risk of developing pancreatitis at any time point up through 5 years of use.[ref][ref][ref][ref]
The Cleveland Clinic now counsels that GLP-1 RA patients should be screened for pancreatitis risk factors, and a lower dose should be used to reduce the risk of pancreatitis.[ref]
Related article: Pancreatitis and Genetic Risk Factors
Gastroparesis:
Case reports tie semaglutide to a risk of gastroparesis (very slow stomach emptying). A large study that compared tirzepatide to semaglutide showed that in non-diabetic, overweight adults, the risk of gastroparesis was much higher in semaglutide recipients compared to tirzepatide. Out of over 17,000 patients on Tirzepatide, 10 reported gastroparesis. With the matched group on semaglutide, 28 reported gastroparesis.[ref][ref]
Diabetic retinopathy:
A couple of clinical trials in people with diabetes linked GLP-1 RA drugs to an increased risk of diabetic retinopathy, while other studies show that the drugs were not associated with the eye disease. A meta-analysis that combined data from systematic reviews and meta-analyses came to the conclusion that there was likely no increase in the risk of diabetic retinopathy from any of the diabetes drugs.[ref] However, this is still a side effect to be aware of the possibility and talk with your doctor about your individual risk.
Related article: Diabetes risk factors
Phamacogenomics and GLP-1 RAs:
There’s an individual variability in treatment response to most medications, including GLP-1 RAs. Different genetic variants are associated with increased or decreased responses to GLP-1 RA drugs for diabetes or weight loss. Researchers have found that differences in the GLP1RA gene, which encodes the GLP-1 receptor, influence how much weight someone is likely to lose on these medications.
Check out your genes below in the genotype report section:
Access this content:
An active subscription is required to access this content.