Key takeaways:
~ Long Covid is the persistence of symptoms after having COVID-19.
~ Fatigue, brain fog, heart rate problems, and breathing issues are the most common symptoms, but other systemic issues can also occur. It is a heterogenous condition.
~ Research on long Covid points towards multiple pathways being involved, which means that the solutions may be different for individuals.
~ Genetic research shows which genes are involved in susceptibility.
SARS-CoV-2: Lingering Effects of a Viral Infection
Before we begin, I want to explain that I’m going to use the term long Covid to keep it simple. Other names for this syndrome include long-haul COVID-19 and Post-acute sequelae of COVID (PASC).
I’ll start with explaining the viral infection process and the body’s immune response to the virus. Understanding the normal immune response to the virus, and what can go wrong, may be key for some long Covid cases. Then I’ll go into the symptoms and likely causes of long Covid.
Viral replication and multiple cell receptors for Covid:
Viruses are non-living strands of RNA or DNA that can only replicate inside a host cell. Essentially, they have to be taken into a cell, and then the host cell replicates the viral genetic material. The viral genes are then translated into their proteins and packaged up by the host cell. The replicated virus then gets released (usually) into the host so that it can infect other cells. Rinse and repeat.
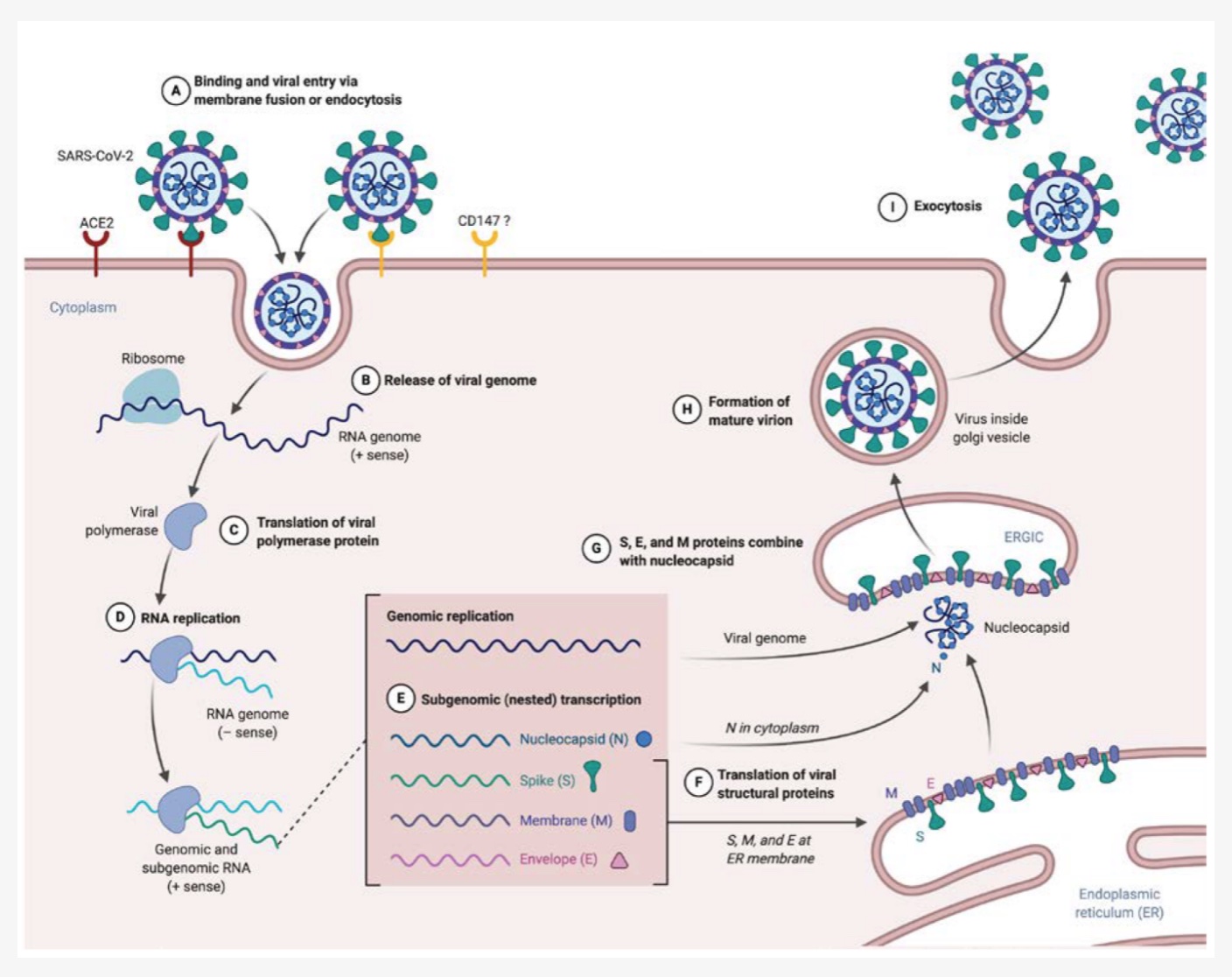
SARS-CoV-2 is a member of the coronavirus family. It is an airborne enveloped, single-stranded RNA virus. “Enveloped” means that the RNA that makes up the virus is packaged within a lipid outer membrane. Other coronaviruses cause the common cold in people, or respiratory diseases, such as kennel cough, in animals. The outside layer of the virus contains spike proteins that bind to a couple of receptors located on the membranes of host cells. The main receptor that the spike protein binds to is the ACE2 receptor.[ref] Additionally, the spike protein can bind to CD147 and NRP1 receptors as well.
The ACE2 receptor is found on the membranes of cells in the lungs, stomach, nose, brain, prostate, testis, placenta, kidney, intestines, and liver. This receptor binds with the spike protein subunits (S1 and S2). Once bound, another host protein, TMPRSS2, cleaves the spike protein off, allowing the release of the viral RNA into the cell.[ref][ref][ref]
The CD147 receptor is a transmembrane glycoprotein of the immunoglobulin superfamily.[ref] Platelets and precursor cells to platelets express the CD147 receptor, and it has been shown that SARS-CoV-2 can enter platelets through this receptor.[ref]
NRP1 encodes neuropilin-1, which is a receptor highly expressed in the brain. SARS-CoV-2 can also bind to NRP1. [ref][ref][ref]
Related article: Neuropilins
As the SARS-CoV-2 virus enters the host cell, it releases its viral RNA into the cell. The host cell then replicates and transcribes the viral RNA into the viral proteins. The viral proteins are taken into the host cell’s endoplasmic reticulum (ER) membrane, where the virus is assembled. It is then translocated to the cell surface to be released via exocytosis to infect other cells.[ref]
Immune response to viruses: Innate and Adaptive
Innate Immune Response:
The innate immune system mounts an immediate response to any foreign pathogens, such as viruses. It is a generic response that is not specific to which virus has invaded. Interferons are produced as one of the first responders in fighting a virus.
For the SARS-CoV-2 virus, the host’s cells recognize patterns associated with viral particles (pattern recognition receptors). This recognition is a general response to any virus with particles that can be recognized.
In SARS-CoV-2 infections, parts of the RNA genome are recognized by Toll-like receptors (TLRs) 3, 7, and 8, as well as MDA5 and RIG-I. Activation of these immune system receptors triggers an antiviral response leading to the induction of a couple of types of interferon as well as proinflammatory cytokines.
When this initial innate immune response is enough to control the infection, it results in the SARS-CoV-2 virus causing mild, cold-like symptoms. A lot of research points to an appropriately strong innate immune response being key to not having severe Covid-19 symptoms. This innate response can be dysregulated in the elderly, leading to susceptibility to viral infections.[ref]
Genetics also plays a role in whether the innate immune response will kick the virus to the curb. People with rare mutations in specific toll-like receptors or interferon-related genes end up with severe Covid-19, even if young and healthy.
Related article: TLR7 common variants and rare mutations
Adaptive Immune Response:
The adaptive immune response takes a bit longer (days), but it is specific to the virus. This additional time is important for the body to produce specific antibodies to bind to the virus and make it non-infective. IgM antibodies are produced in large amounts for a few weeks, and then IgG antibodies can last for a while after an infection to prevent reinfection.
There is a second type of adaptive immune response called cell-mediated immunity. This type of immunity refers to T cells, which are a type of white blood cell that present antigens on their surface. Several different subtypes of T cells are involved in the immune response to SARS-CoV-2, including CD4+ and CD8+ T cells.
In SARS-CoV-2 infections, neutralizing antibodies are produced that bind to the S1 unit of the spike protein and block it from entering cells. In looking at the immune response of people with either asymptomatic, mild, or severe Covid, the researchers found that they all had a similar antibody response. Asymptomatic people, in this particular study, had a better natural killer cell response.[ref]
Research studies on long Covid:
The studies on long Covid are all over the place, some with real scientific inquiry and others simply waxing on about it. Surveys about symptoms make up a lot of the research.
First, I want to address the studies and articles that imply long Covid is “all in your head” and not a real illness. Psychosomatic or psychogenic are terms that have been applied to indicate that it is all psychological and not physiological. Functional neurological disorder is a similar term and a diagnosis that many long Covid patients with brain fog have received.[ref]
For example, this Wall Street Journal from March 2021 implied that long Covid could be all in the patients’ heads. And a Spectator article in 2022 echoes the sentiment. Even some researchers writing published journal articles about long Covid don’t seem completely convinced that it is a real condition. One study put it: “…we should not dismiss their complaints as being all in their head. This may not be true and, even if correct, is not helpful. It is appropriate to acknowledge their distress, get those with fatigue or dyspnea in a structured exercise program, and work with all on symptomatic relief.”[ref]
This echoes a lot of research articles about ME/CFS from the 80s and 90s, which inferred the fatigue was psychosomatic and the patients should be prescribed antidepressants.[ref][ref][ref]
The “it’s all in your head” idea has now been thoroughly debunked with a multitude of studies showing significant, physiological and cellular changes occurring in both long Covid and ME/CFS.[ref][ref]
Defining Long Covid:
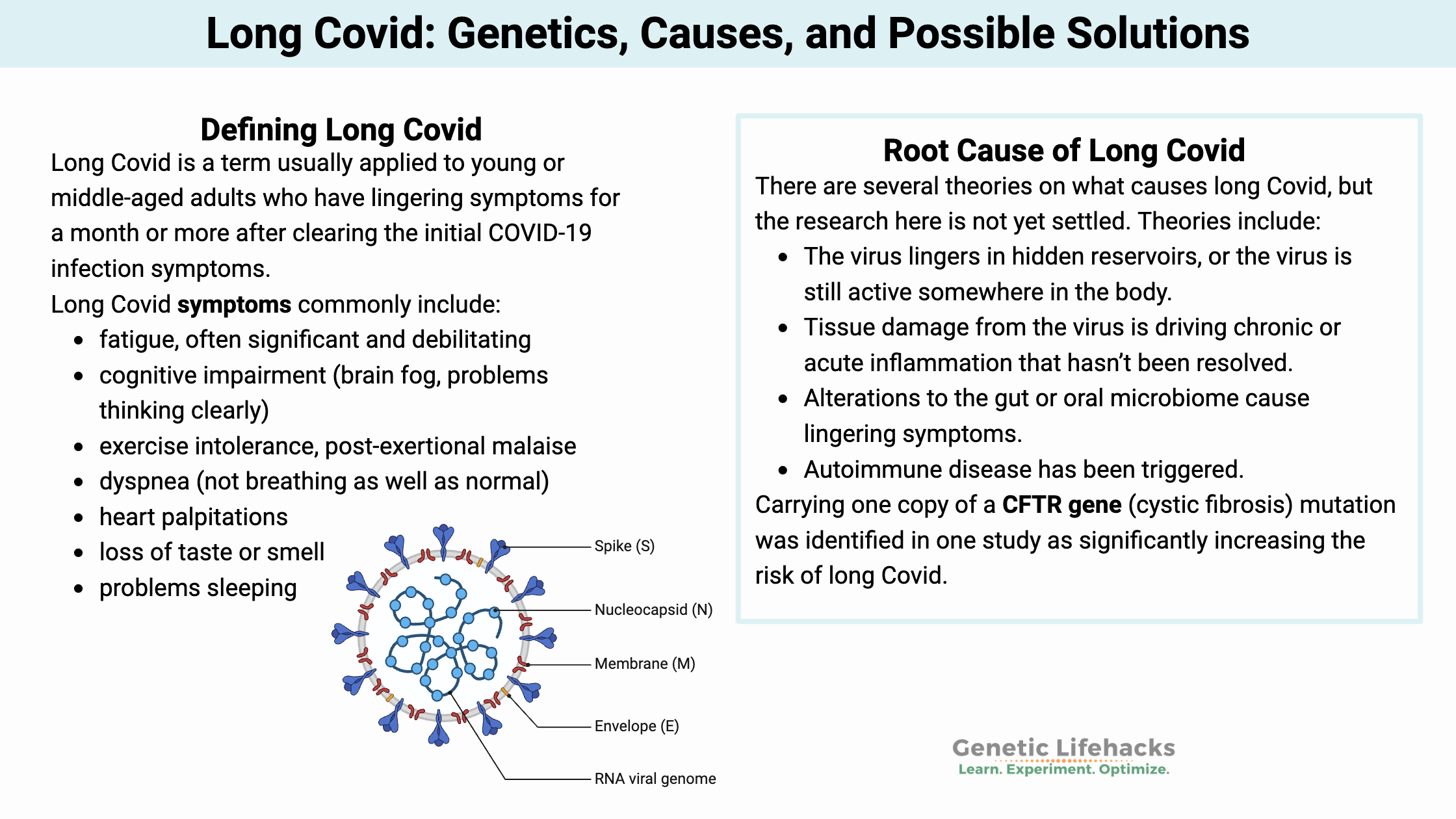
Long Covid is a term usually applied to young or middle-aged adults who have lingering symptoms for a month or more after clearing the initial COVID-19 infection symptoms.[ref][ref][ref] Older people who had severe COVID-19 and ended up in the ICU often have long-term decreased functional capacity due to cell damage. For example, lung problems persist for many months for COVID-19 patients after the ICU stay. [ref]
Long Covid symptoms commonly include:
- fatigue, often significant and debilitating
- cognitive impairment (brain fog, problems thinking clearly)
- exercise intolerance, post-exertional malaise
- dyspnea (not breathing as well as normal)
- heart palpitations
- loss of taste or smell
- problems sleeping
- hair loss
- headache
- gynecological problems
- hyperhidrosis (sweating a lot)
Post-infectious symptoms aren’t new or unexpected:
It’s important to point out that the long-term effects of viral infections aren’t new, but they are specific to the virus. Here are a few examples of lingering viral effects:[ref][ref][ref][ref][ref]
- Hearing loss is a serious complication after having mumps.
- Zika virus and cytomegalovirus can have significant long-term effects on children if exposed as an infant.
- Some SARS and MERS patients had long-lasting significant impairments for years after their infections.
- Post-viral syndrome, which usually involves significant fatigue, muscle pain, brain fog, etc., occurs with the Coxsackie virus, brucellosis, poliovirus, viral meningitis, Ross River virus, and Epstein-Barr virus.
- Poliovirus can cause long-term disability.
- Herpes simplex 1 (the cause of cold sores) is linked to neurodegenerative disorders, such as Alzheimer’s.
Importantly, though, lingering viral symptoms are neither well studied nor easily treated.
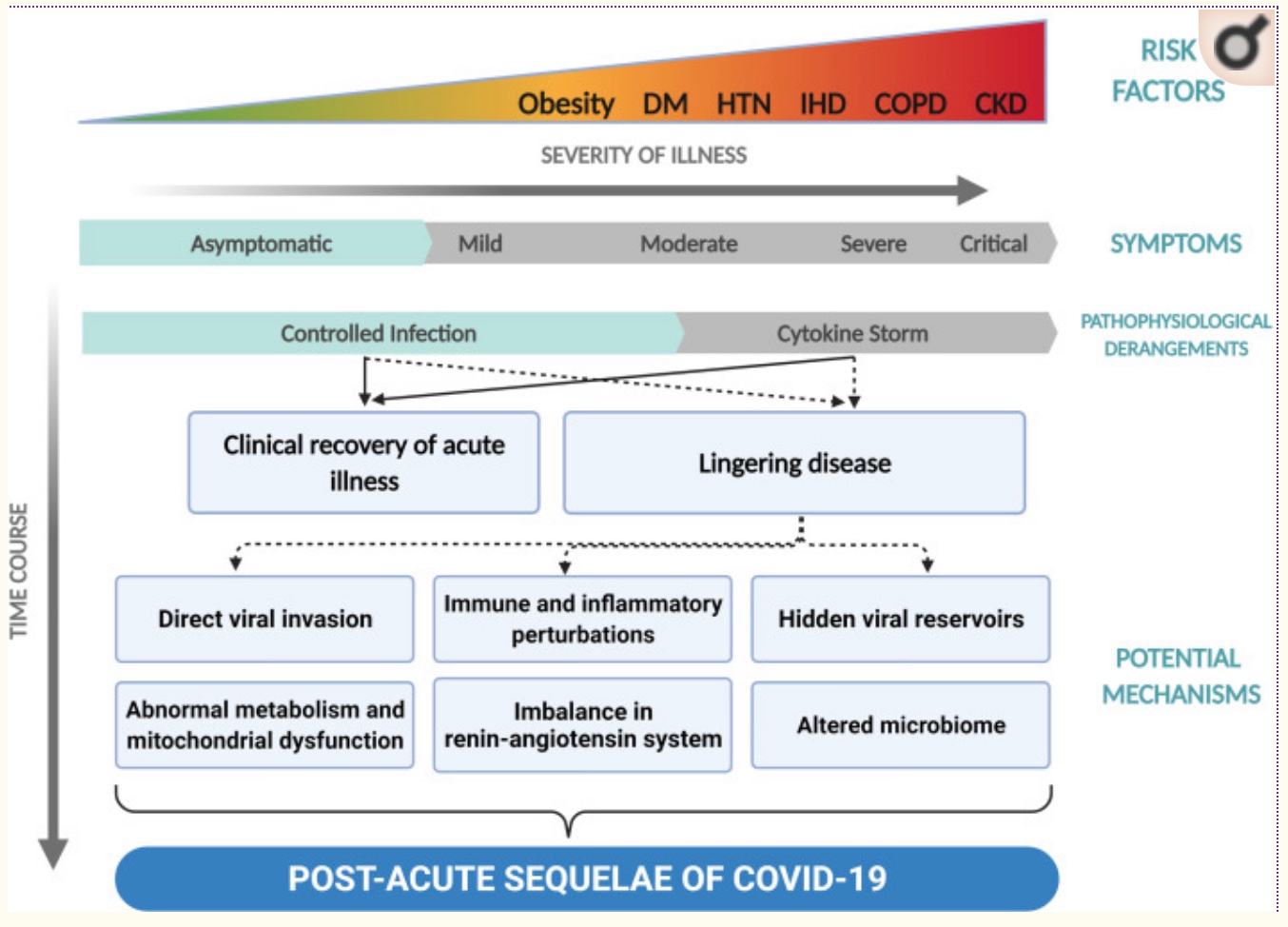
Getting specific on the root cause of long Covid:
With the wide range of symptoms, different causes could be impacting individuals independently.
There are several theories on what is going on with long Covid, and the research here is not yet settled. Theories include:[ref]
- The virus lingers in hidden reservoirs, or the virus is still active somewhere in the body.
- Tissue damage from the virus is driving chronic or acute inflammation that hasn’t been resolved.
- Alterations to the gut or oral microbiome cause lingering symptoms.
- Autoimmune disease has been triggered.
Let’s dig into some of the research findings:
Muscle and mitochondrial abnormalities in post-exertional malaise (PEM):
A January 2024 study explains a lot about what is occurring at a cellular level in people who have exercise intolerance or post-exertional malaise (PEM) after Covid. (Note that post-exertional malaise also includes mental exertion.)
The study found significant physiological changes in muscle tissue in long Covid patients with PEM. First, they found that exercise or muscle exertion causes amyloid-containing deposits to form in the muscles. Additionally, there were systemic metabolic disturbances in the way the skeletal muscle cells worked. The long Covid patients had lower oxidative phosphorylation capacity, meaning their mitochondria weren’t producing enough ATP for cellular energy. The long Covid patients’ cells had a normal number of mitochondria, but they weren’t functioning like they should. In addition, there was evidence of severe muscle tissue damage with blunted T-cell response.[ref – open access, worth reading in full]
Also important is what the study didn’t find. The researchers didn’t find that the muscles had increased breakdown products or higher cortisol levels. They also didn’t find activation of damaged protein systems (DAMP). While there was evidence of SARS-CoV-2 nucleocapsid protein in the muscle, the levels did not differ from the healthy controls.
Complement system activation:
The complement system is part of the innate immune response. It can be activated by pathogens, or it can respond to damaged cells.[ref]
A recent proteomics study of 268 long Covid patient samples found that there was ongoing activation of the alternative and classical complement pathways. There were also increased antibodies against several herpesviruses.[ref]
Related article: Mannose-binding lectin genes
Lingering spike protein:
Research shows that the spike protein alone (without the virus) causes an immune response, including abnormal clotting.[ref][ref]
- A recent study found that CD16+ monocytes had persistent spike (S1) proteins in patients with PASC (Post-acute sequelae of COVID) long after the infection should have cleared. CD16+ monocytes interact with the endothelium and with platelets.[ref]
- Another study found similar results with spike protein fragments found in a long Covid patient’s monocytes 15 months post-infection.[ref]
- The spike protein is also given in vaccines. These studies on PASC/long Covid patients don’t include information on whether the patients were also vaccinated. A recent study published in Cell shows that the spike protein mRNA is present in some lymph nodes eight weeks after vaccination.[ref]
Reactivation of Epstein-Barr Virus:
A study of patients with long Covid showed that 67% had positive Epstein-Barr titers showing a reactivation of the virus. (Only 10% of the healthy control group had active Epstein-Barr virus titers.) The study concludes: “These findings suggest that many long COVID symptoms may not be a direct result of the SARS-CoV-2 virus but may be the result of COVID-19 inflammation-induced EBV reactivation.”[ref]
Related article: Epstein-Barr Virus: Genetic Risks, Reactivation, and Chronic Illnesses
Another recent study showed that people who had reactivated EBV before getting Covid were twice as likely to develop long Covid. Interestingly, having had cytomegalovirus was protective against the neurocognitive symptoms of long Covid.[ref]
Persistent clotting problems and inflammation:
A study looked at plasma samples from long Covid /PASC patients and found large anomalous deposits (microclots). The clots were resistant to fibrinolysis. Additionally, the researchers noted various inflammatory cytokines that were elevated in long Covid patients, including “α(2)-antiplasmin (α2AP), various fibrinogen chains, as well as Serum Amyloid A (SAA)”.[ref] Serum amyloid A is an acute-phase protein indicating active inflammation.
Post-COVID-19 Tachycardia Syndrome:
Researchers have identified that a subset of patients end up with heart palpitations or tachycardia that persists for 3+ months after Covid. This abnormality can also include POTS (postural tachycardia syndrome) patients. Interestingly, the researchers did not find Holter ECG monitoring matched with reported symptoms. The researchers think that direct and indirect damage to the heart from the viral infection may lead to post-Covid tachycardia syndrome. Additional causes are theorized to include persistent lung injury, persistent fevers, pain, anxiety, or depression.[ref]
Related article: POTS, genes
Altered microbiome:
Changes to the gut microbiome or oral microbiome due to SARS-CoV-2 could lead to immune dysregulation and chronic inflammation.
- A study of COVID-19 patients looked at changes to the gut microbiome in the participants who still had lingering symptoms at 6 months (mainly fatigue, brain fog, and hair loss). The gut microbiome of long Covid patients showed persistent differences. “Butyrate-producing bacteria, including Bifidobacterium pseudocatenulatum and Faecalibacterium prausnitzii showed the largest inverse correlations with PACS at 6 months.”[ref]
- A study of the oral microbiome in patients with COVID-19 showed that those who developed long Covid had significant differences in the oral microbiome. The long-covid patients had “significantly higher abundances of microbiota that induced inflammation… The oral microbiome of patients with long COVID was similar to that of patients with chronic fatigue syndrome.”[ref]
Persistent Immune System Changes:
A study of upregulated genes in people with long Covid showed that platelet-related pathways were downregulated and genes involved in transcription, translation, and the cell cycle were upregulated. The downregulation of platelet-related genes (e.g. platelet factor 4, coagulation factor XIIII) may correlate to thrombocytopenia (low platelet count) seen in COVID-19 patients – and be a source of fatigue. Interestingly, the researchers also identified interferon-related genes as being downregulated.[ref]
Systemic inflammation:
Elevated inflammatory cytokines are found in people with long Covid.
- A PET/CT study of people with long Covid showed that systemic inflammation might be the underlying cause. The imaging study found hypometabolism in the brain, which is typical of whole-body inflammatory changes. It means that regions of the brain are literally not getting enough energy, and the cause is likely inflammation in the body.[ref] What the study doesn’t show is whether the systemic inflammation is due to lack of resolution of inflammation, persistence of viral infection, or sterile inflammation due to cell death from the inflammatory response to the virus.
- TNF-alpha and interferon-gamma: Another preprint describes the differences between people who fully recovered from Covid and people with long Covid. “participants with respiratory PASC had up to 34-fold higher frequencies of IFN-γ- and TNF-α-producing SARS-CoV-2-specific CD4+ and CD8+ T cells in peripheral blood and elevated levels of plasma CRP and IL-6.”[ref]
Spike protein in the brain:
Some researchers theorize that the spike protein causes neurological issues in long Covid. This researcher points to indirect evidence of the spike protein being released and possibly crossing the blood-brain barrier. Animal studies show that the spike protein alone can cross the blood-brain barrier.[ref][ref]
Persistence of virus:
Many viruses can stick around in the body, usually in a latent form, for a long time. For example, the varicella-zoster virus that causes chickenpox lies dormant in the nervous system and can reactivate as shingles in adulthood. The question is whether long Covid symptoms are caused by the SARS-CoV-2 virus still lingering, at low levels, somewhere in the body.
- A study on viral persistence shows that people with long Covid have higher cytokine levels such as TNF-alpha and IL-6. The researchers thought that this might indicate persistent infection.[ref]
- Lung tissue biopsy showed the persistence of SARS-CoV-2 RNA in the lungs more than 100 days after a mild infection.[ref]
- Intestinal biopsies of asymptomatic individuals also showed SARS-CoV-2 RNA in half the patients at four months post-infection.[ref]
- A trial using sweat samples and dogs that can sniff Covid found that about half the long Covid patients tested (sniffed) positive for viral infection.[ref]
Autoimmune antibodies:
A study of patients with severe COVID-19 found that 34% of them were positive for antinuclear antibodies, another 34% were positive for anti-β2GPI antibodies, and several other antibodies were also determined. Importantly, none of the patients in the study had previously been diagnosed with a rheumatic disease (which would be a source of these specific autoimmune-related antibodies).[ref][ref] The question, though, is how long the autoimmune antibodies last after an illness. It is thought that the low-level autoimmune antibodies will likely fade away fairly quickly after the illness.[ref]
Epigenetic changes and gene expression:
New research suggests that SARS-CoV-2 infection causes changes in gene expression in important ways. Infection has been shown to cause changes in the chromatin structure of DNA, which alters which genes are accessible for translation into proteins.
One study found that there is an upregulation of pro-inflammatory cytokine production and cell adhesion proteins. It also found that there were overall alterations in transcription factors.[ref]
More research is needed here to understand whether some of the changes in gene expression are continuing after the active infection and possibly causing long Covid symptoms. Epigenetic changes are seen for up to a year in hematopoietic stem and progenitor cells, which produce immune cells (T cells) and red blood cells. [ref][ref]
Genotype report: Long Covid
Lifehacks: Possible solutions for long Covid
Related Articles and Topics:
TLR7: Susceptibility to COVID-19
A genetic variant related to Covid severity.
Genetic susceptibility to viruses
Your genetic variants shape your immune system and give you superpowers against some pathogens – and perhaps more susceptible to others.
Vitamin D, Genes, and Your Immune System
Vitamin D is more than just a ‘vitamin’. It is actually a hormone that is essential to so many processes in your body – including your immune system.
Acute Respiratory Distress Syndrome Genes
This is article explains what happens to the body in ARDS, and it goes into the genetic variants that increase or decrease the risk of ARDS (due to all causes – not just COVID-19). ARDS is a ‘syndrome’, and thus a collection of symptoms rather than a disease.
References:
“Axcella Commences Trial of AXA1125 for Long Covid-19 Treatment.” Clinical Trials Arena, 27 Oct. 2021, https://www.clinicaltrialsarena.com/news/axcella-trial-long-covid-treatment/.
Barrett, Tessa J., et al. “Platelets Contribute to Disease Severity in COVID‐19.” Journal of Thrombosis and Haemostasis, vol. 19, no. 12, Dec. 2021, pp. 3139–53. PubMed Central, https://doi.org/10.1111/jth.15534.
Carsetti, Rita, et al. “Different Innate and Adaptive Immune Responses to SARS-CoV-2 Infection of Asymptomatic, Mild, and Severe Cases.” Frontiers in Immunology, vol. 11, Dec. 2020, p. 610300. PubMed Central, https://doi.org/10.3389/fimmu.2020.610300.
Ceulemans, Laurens J., et al. “Persistence of SARS-CoV-2 RNA in Lung Tissue after Mild COVID-19.” The Lancet. Respiratory Medicine, vol. 9, no. 8, Aug. 2021, pp. e78–79. PubMed Central, https://doi.org/10.1016/S2213-2600(21)00240-X.
Devine, Jeremy. “Opinion | The Dubious Origins of Long Covid.” Wall Street Journal, 22 Mar. 2021. www.wsj.com, https://www.wsj.com/articles/the-dubious-origins-of-long-covid-11616452583.
Eskandari-Nasab, Ebrahim, et al. “Meta-Analysis of Risk Association Between Interleukin-17A and F Gene Polymorphisms and Inflammatory Diseases.” Journal of Interferon & Cytokine Research: The Official Journal of the International Society for Interferon and Cytokine Research, vol. 37, no. 4, Apr. 2017, pp. 165–74. PubMed, https://doi.org/10.1089/jir.2016.0088.
Feng, Bo, et al. “Association of Tumor Necrosis Factor α -308G/A and Interleukin-6 -174G/C Gene Polymorphism with Pneumonia-Induced Sepsis.” Journal of Critical Care, vol. 30, no. 5, Oct. 2015, pp. 920–23. PubMed, https://doi.org/10.1016/j.jcrc.2015.04.123.
Glynne, Paul, et al. “Long COVID Following Mild SARS-CoV-2 Infection: Characteristic T Cell Alterations and Response to Antihistamines.” Journal of Investigative Medicine, vol. 70, no. 1, Jan. 2022, pp. 61–67. jim.bmj.com, https://doi.org/10.1136/jim-2021-002051.
Gold, Jeffrey E., et al. “Investigation of Long COVID Prevalence and Its Relationship to Epstein-Barr Virus Reactivation.” Pathogens, vol. 10, no. 6, June 2021, p. 763. PubMed Central, https://doi.org/10.3390/pathogens10060763.
Grandjean, Dominique, et al. Screening for SARS-CoV-2 Persistence in Long COVID Patients Using Sniffer Dogs and Scents from Axillary Sweats Samples. medRxiv, 12 Jan. 2022, p. 2022.01.11.21268036. medRxiv, https://www.medrxiv.org/content/10.1101/2022.01.11.21268036v1.
Hoffer, Edward P. “Long COVID: Does It Exist? What Is It? We Can We Do For Sufferers?” The American Journal of Medicine, vol. 134, no. 11, Nov. 2021, pp. 1310–11. PubMed Central, https://doi.org/10.1016/j.amjmed.2021.05.023.
Lebeau, Grégorie, et al. “Deciphering SARS-CoV-2 Virologic and Immunologic Features.” International Journal of Molecular Sciences, vol. 21, no. 16, Jan. 2020, p. 5932. www.mdpi.com, https://doi.org/10.3390/ijms21165932.
Littlefield, Katherine M., et al. SARS-CoV-2-Specific T Cells Associate with Reduced Lung Function and Inflammation in Pulmonary Post-Acute Sequalae of SARS-CoV-2. bioRxiv, 15 Feb. 2022, p. 2022.02.14.480317. bioRxiv, https://www.biorxiv.org/content/10.1101/2022.02.14.480317v1.
Liu, Qin, et al. “Gut Microbiota Dynamics in a Prospective Cohort of Patients with Post-Acute COVID-19 Syndrome.” Gut, Jan. 2022, p. gutjnl-2021-325989. PubMed Central, https://doi.org/10.1136/gutjnl-2021-325989.
“Long Covid-19: Drug Trial Results to Watch in 2022.” Clinical Trials Arena, 25 Jan. 2022, https://www.clinicaltrialsarena.com/analysis/long-covid-19-drug-trial-results-to-watch-in-2022/.
MacIntyre, Elaina A., et al. “GSTP1 and TNF Gene Variants and Associations between Air Pollution and Incident Childhood Asthma: The Traffic, Asthma and Genetics (TAG) Study.” Environmental Health Perspectives, vol. 122, no. 4, Apr. 2014, pp. 418–24. PubMed, https://doi.org/10.1289/ehp.1307459.
Majumder, Poulami, et al. “Association of Tumor Necrosis Factor-α (TNF-α) Gene Promoter Polymorphisms with Aggressive and Chronic Periodontitis in the Eastern Indian Population.” Bioscience Reports, vol. 38, no. 4, Aug. 2018, p. BSR20171212. PubMed, https://doi.org/10.1042/BSR20171212.
Patterson, Bruce, et al. Targeting the Monocytic-Endothelial-Platelet Axis with Maraviroc and Pravastatin as a Therapeutic Option to Treat Long COVID/ Post-Acute Sequelae of COVID (PASC). 2 Mar. 2022. Research Square, https://www.researchsquare.com/article/rs-1344323/v1.
Peluso, Michael J., et al. Markers of Immune Activation and Inflammation in Individuals with Post-Acute Sequelae of SARS-CoV-2 Infection. medRxiv, 11 July 2021, p. 2021.07.09.21260287. medRxiv, https://www.medrxiv.org/content/10.1101/2021.07.09.21260287v1.
Pretorius, Etheresia, et al. “Persistent Clotting Protein Pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) Is Accompanied by Increased Levels of Antiplasmin.” Cardiovascular Diabetology, vol. 20, Aug. 2021, p. 172. PubMed Central, https://doi.org/10.1186/s12933-021-01359-7.
Ramakrishnan, Rakhee K., et al. “Unraveling the Mystery Surrounding Post-Acute Sequelae of COVID-19.” Frontiers in Immunology, vol. 12, June 2021, p. 686029. PubMed Central, https://doi.org/10.3389/fimmu.2021.686029.
Ricci, Daniela, et al. “Innate Immune Response to SARS-CoV-2 Infection: From Cells to Soluble Mediators.” International Journal of Molecular Sciences, vol. 22, no. 13, June 2021, p. 7017. PubMed Central, https://doi.org/10.3390/ijms22137017.
Röltgen, Katharina, et al. “Immune Imprinting, Breadth of Variant Recognition, and Germinal Center Response in Human SARS-CoV-2 Infection and Vaccination.” Cell, vol. 0, no. 0, Jan. 2022. www.cell.com, https://doi.org/10.1016/j.cell.2022.01.018.
Rowe, Regina K., and Emma L. Mohr. “Special Issue ‘Pediatric Viral Infection Long-Term Consequences.’” Viruses, vol. 14, no. 2, Feb. 2022, p. 343. www.mdpi.com, https://doi.org/10.3390/v14020343.
Ryan, Feargal J., et al. “Long-Term Perturbation of the Peripheral Immune System Months after SARS-CoV-2 Infection.” BMC Medicine, vol. 20, Jan. 2022, p. 26. PubMed Central, https://doi.org/10.1186/s12916-021-02228-6.
Ryu, Jae Kyu, et al. SARS-CoV-2 Spike Protein Induces Abnormal Inflammatory Blood Clots Neutralized by Fibrin Immunotherapy. bioRxiv, 13 Oct. 2021, p. 2021.10.12.464152. bioRxiv, https://www.biorxiv.org/content/10.1101/2021.10.12.464152v1.
Saheb Sharif-Askari, Narjes, et al. “Enhanced Expression of Autoantigens During SARS-CoV-2 Viral Infection.” Frontiers in Immunology, vol. 12, June 2021, p. 686462. PubMed Central, https://doi.org/10.3389/fimmu.2021.686462.
Sapkota, Hem Raj, and Arvind Nune. “Long COVID from Rheumatology Perspective — a Narrative Review.” Clinical Rheumatology, vol. 41, no. 2, 2022, pp. 337–48. PubMed Central, https://doi.org/10.1007/s10067-021-06001-1.
Sollini, Martina, et al. “Long COVID Hallmarks on [18F]FDG-PET/CT: A Case-Control Study.” European Journal of Nuclear Medicine and Molecular Imaging, vol. 48, no. 10, 2021, pp. 3187–97. PubMed Central, https://doi.org/10.1007/s00259-021-05294-3.
Ståhlberg, Marcus, et al. “Post-COVID-19 Tachycardia Syndrome: A Distinct Phenotype of Post-Acute COVID-19 Syndrome.” The American Journal of Medicine, vol. 134, no. 12, Dec. 2021, pp. 1451–56. PubMed Central, https://doi.org/10.1016/j.amjmed.2021.07.004.
Stappers, M. H. T., et al. “Polymorphisms in Cytokine Genes IL6, TNF, IL10, IL17A and IFNG Influence Susceptibility to Complicated Skin and Skin Structure Infections.” European Journal of Clinical Microbiology & Infectious Diseases: Official Publication of the European Society of Clinical Microbiology, vol. 33, no. 12, Dec. 2014, pp. 2267–74. PubMed, https://doi.org/10.1007/s10096-014-2201-0.
Theoharides, Theoharis C. “Could SARS-CoV-2 Spike Protein Be Responsible for Long-COVID Syndrome?” Molecular Neurobiology, Jan. 2022, pp. 1–12. PubMed Central, https://doi.org/10.1007/s12035-021-02696-0.
Tirelli, U., et al. “Fatigue in Post-Acute Sequelae of SARS-CoV2 (PASC) Treated with Oxygen-Ozone Autohemotherapy – Preliminary Results on 100 Patients.” European Review for Medical and Pharmacological Sciences, vol. 25, no. 18, Sept. 2021, pp. 5871–75. PubMed, https://doi.org/10.26355/eurrev_202109_26809.
Vlachoyiannopoulos, Panayiotis G., et al. “Autoantibodies Related to Systemic Autoimmune Rheumatic Diseases in Severely Ill Patients with COVID-19.” Annals of the Rheumatic Diseases, vol. 79, no. 12, Dec. 2020, pp. 1661–63. ard.bmj.com, https://doi.org/10.1136/annrheumdis-2020-218009.
Walitt, Brian, and Elizabeth Bartrum. “A Clinical Primer for the Expected and Potential Post-COVID-19 Syndromes.” Pain Reports, vol. 6, no. 1, Feb. 2021, p. e887. PubMed Central, https://doi.org/10.1097/PR9.0000000000000887.
Wang, Ke, et al. “CD147-Spike Protein Is a Novel Route for SARS-CoV-2 Infection to Host Cells.” Signal Transduction and Targeted Therapy, vol. 5, no. 1, Dec. 2020, pp. 1–10. www.nature.com, https://doi.org/10.1038/s41392-020-00426-x.
Wu, Jun-Cang, et al. “Gene Polymorphisms and Circulating Levels of the TNF-Alpha Are Associated with Ischemic Stroke: A Meta-Analysis Based on 19,873 Individuals.” International Immunopharmacology, vol. 75, Oct. 2019, p. 105827. PubMed, https://doi.org/10.1016/j.intimp.2019.105827.
Yong, Shin Jie. “Long COVID or Post-COVID-19 Syndrome: Putative Pathophysiology, Risk Factors, and Treatments.” Infectious Diseases (London, England), pp. 1–18. PubMed Central, https://doi.org/10.1080/23744235.2021.1924397. Accessed 2 Mar. 2022.
Yucesoy, Berran, et al. “Genetic Variants in TNFα, TGFB1, PTGS1 and PTGS2 Genes Are Associated with Diisocyanate-Induced Asthma.” Journal of Immunotoxicology, vol. 13, no. 1, 2016, pp. 119–26. PubMed, https://doi.org/10.3109/1547691X.2015.1017061.