Key takeaways for histamine intolerance:
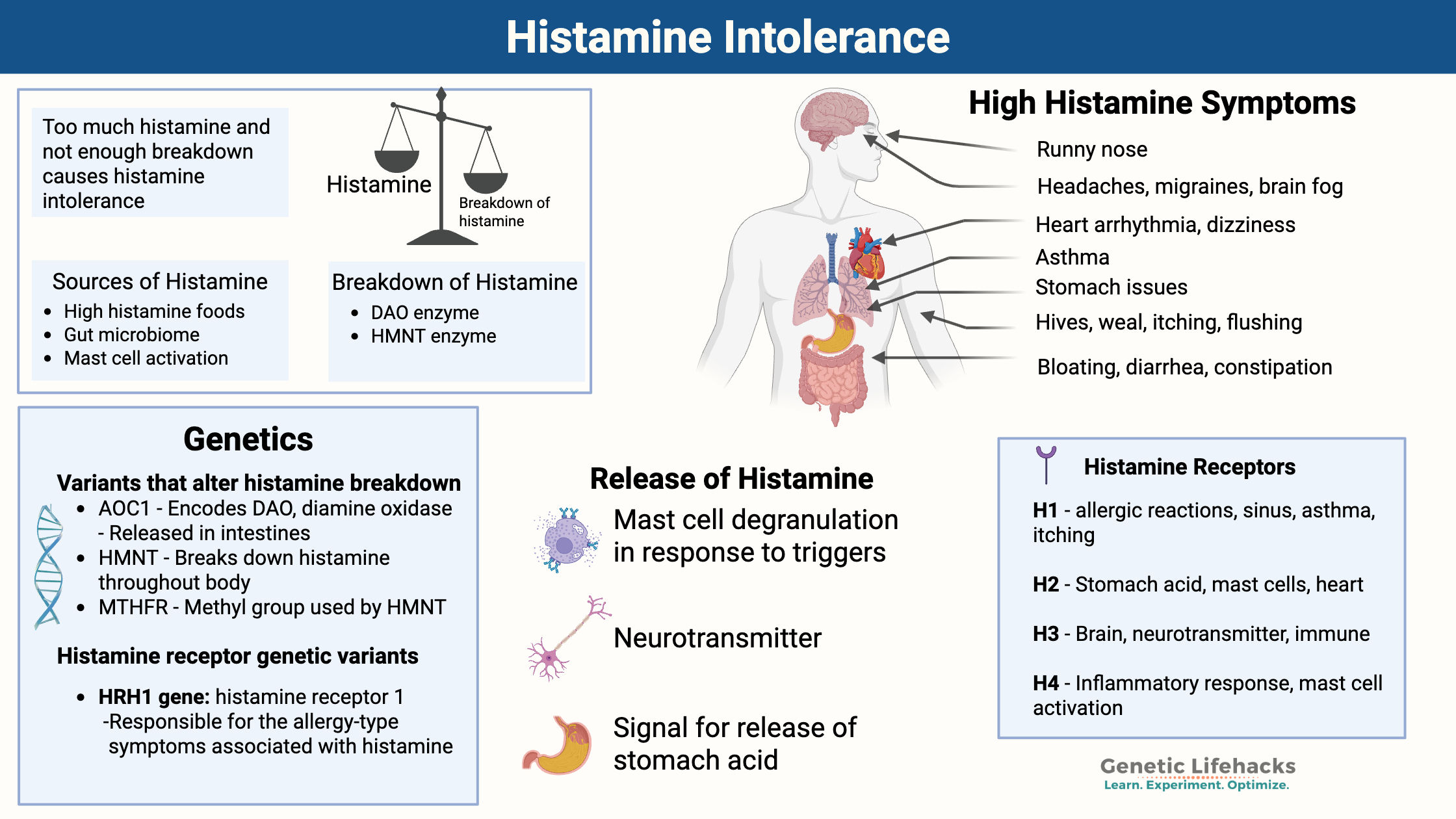
~ Histamine intolerance is caused by an imbalance of too much histamine compared to your capacity to break it down. This can be due to excess histamine from food, producing too much histamine, or not being able to break it down efficiently.
~ Genetic variants (SNPs) in the genes encoding DAO and HNMT can reduce enzyme activity, which increases susceptibility to histamine intolerance.
~ Histamine intolerance can cause a wide range of symptoms including: Headaches, migraines, anxiety, irritability, brain fog, acid reflux, nausea, bloating, diarrhea, arrhythmia, dizziness, sinus drainage, hives, itching, flushing, insomnia, and early waking.
~ Understanding your genetic variants can help you target dietary changes, lifestyle modifications, and natural supplements to help with histamine intolerance symptoms.
Want more information on Histamine Intolerance? I wrote the book on it! Get your copy of Histamine Lifehacks eBook here – $4.99 Sale.
Members will see their genotype report below, plus additional solutions in the Lifehacks section. Consider joining today.
Histamine Intolerance: Causes, symptoms, genetics, and solutions
Do you deal with sinus drainage after you eat? Periodic itching and hives? Migraines, irritability, anxiety, and brain fog? The weird and seemingly unrelated symptoms of histamine intolerance can drive you nuts trying to figure out the root cause.
Histamine is a biogenic amine that plays many roles in the body. Histamine’s many functions include:
- causes the symptoms in allergic reactions,
- acts within our immune defense system,
- dilates blood vessels (vasodilatation)
- acts as a neurotransmitter
- works as a signaling molecule in the stomach to release acid
While most of us think of histamine in relation to allergies, histamine is a vital part of how your body normally works. The key is that you want histamine in the right amount. Balanced histamine levels are essential.
Histamine intolerance results from an imbalance between histamine intake/production and your body’s ability to break it down. Symptoms of high histamine levels are diverse and can affect the head, mood, gut, heart, skin, and sleep.
| Body System | Common Symptoms |
|---|---|
| Head | Headaches, migraines |
| Mood/Brain | Anxiety, irritability, brain fog |
| Stomach | Acid reflux, nausea, stomach pain |
| Intestines | Bloating, diarrhea, constipation |
| Heart | Arrhythmia, dizziness |
| Sinuses | Drainage, congestion |
| Skin | Hives, itching, flushing, dandruff |
| Sleep | Insomnia, early waking |
| Bladder | Urgency, frequency, and leakage |
People with histamine intolerance usually have several of the symptoms above, but likely won’t have all of the symptoms.[ref]
Here’s an overview of what we are going to cover in this article:
Causes of histamine intolerance:
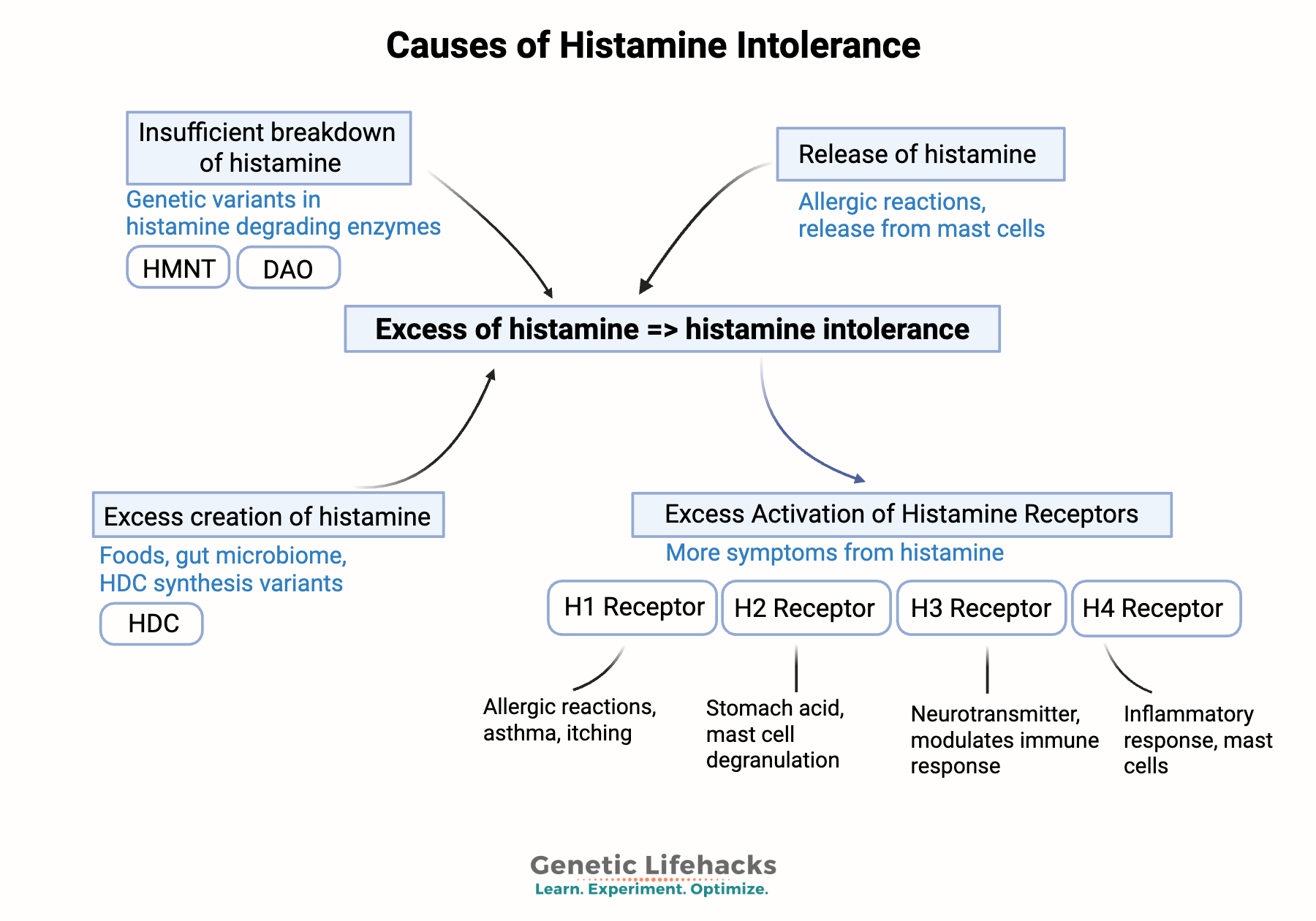
There are two main reasons someone might develop histamine intolerance:
- Impaired histamine breakdown due to deficiencies in the DAO and HNMT enzymes.
– and/or- - Excess histamine is being produced, often due to gut dysbiosis, leaky gut, mast cells, HDC variants, or chronic exposure to allergens.
1) Impaired histamine breakdown:
There are two ways your body breaks down and clears histamine: the DAO enzyme or the HMNT enzyme.[ref]
| Enzyme Name | Gene | Location/Action | Main Role |
|---|---|---|---|
| Diamine Oxidase (DAO) | AOC1 | Primarily in the small intestine | Breaks down histamine from foods and gut bacteria [ref] |
| Histamine N-methyltransferase (HNMT) | HNMT | Throughout the body, especially the brain | Breaks down histamine produced by your cells [ref] |
Diamine oxidase (DAO) enzyme: breaking down and inactivating histamine in the gut
Diamine oxidase is encoded by the AOC1 gene. It is primarily produced in the intestines to break down histamine from foods and histamine created by intestinal bacteria. Some species of bacteria in the gut, including those from some probiotics or fermented foods, can add to your body’s histamine level.
- People with histamine intolerance show altered gut microbiome composition as well as elevated levels of zonulin, which regulates tight junctions in the intestines. Excess zonulin causes ‘leaky gut’. [ref]
- A recent study of histamine intolerance patients found that they had “a significantly higher abundance of histamine-secreting bacteria…”[ref]
Histamine plus other biogenic amines: The DAO enzyme is also used by the body to break down other biogenic amines, including tyramine, putrescine, cadaverine, spermidine, and spermine. High levels of other biogenic amines can reduce the ability of DAO to break down histamine.[ref]
DAO degrades histamine into imidazole acetaldehyde, which is then quickly oxidized into imidazole acetic acid.[ref]
Histamine + [DAO enzyme] → Imidazole acetaldehyde + NH3+H2O2
HMNT enzyme: inactivating histamine produced in your cells
In addition to its role in allergic response and in the gut, histamine is produced and functions as a neurotransmitter within the brain and nervous system. The primary enzyme responsible for breaking down histamine in the central nervous system is histamine N-methyltransferase (HNMT). While DAO can also circulate in the periphery, HNMT is the only enzyme that breaks down histamine as a neurotransmitter in the central nervous system and throughout the body.
Genetic variants in HNMT show that brain histamine levels are linked to:[ref][ref][ref]
- Migraines
- ADHD
- Neurodegenerative disorders, such as Parkinson’s disease.
- Cognition
HNMT is active throughout the body, not just in the brain. Genetically decreased HNMT is also linked to:
- Atopic dermatitis or eczema
- Allergies
When HNMT breaks down histamine, it converts it into N-methylhistamine, which is unable to bind to histamine receptors. This reaction uses a methyl group, which ties into the methylation cycle (more on this later). The N-methylhistamine is further broken down with the MAO-B enzyme, forming N-methylimidazole acetaldehyde.[ref]
Histamine (from body cells) → [HNMT enzyme] → N-methylhistamine → [MAO-B enzyme] → N-methylimidazole acetaldehyde
Note that the HNMT enzyme (histamine N-methyltransferase) facilitates the transfer of a methyl group in the breakdown of histamine through this pathway. Thus, having sufficient methyl groups available is important. This ties histamine metabolism into the methylation cycle and MTHFR variants. (more on this in the Genotype report and Lifehacks sections)
2) Excess Histamine Synthesis: The other side of the equation
Histamine is synthesized in cells and then used in multiple ways throughout the body.
Synthesis:
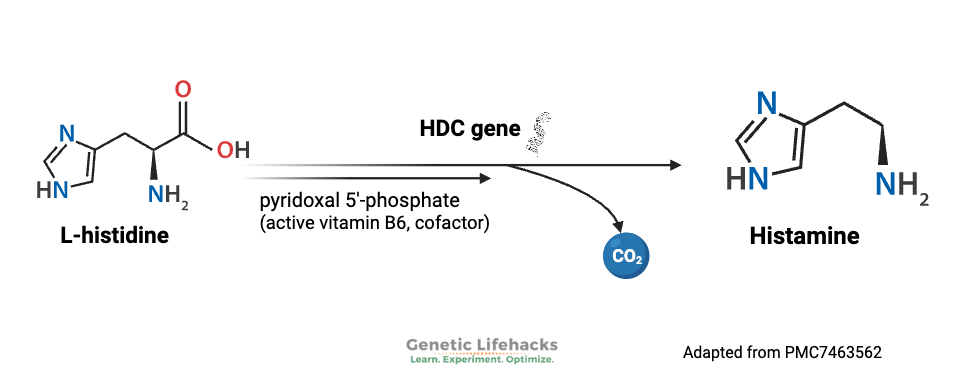
Histamine is made from the amino acid histidine. Histidine is an essential amino acid, meaning humans cannot make it in our bodies and must obtain it from our food. Histidine can be used in the body for several different purposes, including histamine production, metal ion chelation, and neutralizing ROS.
Histidine decarboxylase (HDC gene) is an enzyme that catalyzes the reaction of histidine into histamine. It does this inside various cell types, including creating histamine in large amounts in mast cells.
What happens with too little histamine in the brain?
Without enough histidine decarboxylase (HDC), animals show behavior that resembles Tourette syndrome.
Genetic studies show that people with Tourette’s (vocal and motor tics) can have HDC mutations as a cause. The loss of histamine in the basal ganglia region of the brain causes too much dopamine, resulting in tics.[ref]
Too much histamine affects the heart:
Histamine is also essential in how the heart muscle functions. Too much histamine there can be detrimental, and people with chronic heart failure have higher average plasma histamine levels. In fact, a genetic variant in the HDC gene that reduces histamine levels is linked to a significantly decreased risk of chronic heart failure.[ref]
Additionally, clinical trials show that blocking the H2 receptor is beneficial for chronic heart failure. Famotidine improved cardiac symptoms and ventricular remodeling. In the heart, histamine increases the force of contraction, and even as far back as 1913, histamine has been known to induce heart arrhythmias.[ref][ref]
Mast cells and histamine release:
Mast cells are a type of immune cell that creates and stores histamine. They are found in most tissues in the body, especially in areas of the body exposed to the outside world. Allergens cause mast cells to burst or degranulate and release histamine. Large numbers of mast cells are found in the skin, bronchial tree mucosa, and intestinal mucosa. In addition to allergens, viruses, bacteria, and fungi also activate mast cells.[ref]
Mast cells are also the primary source of histamine released to circulate in the body.[ref]
The release of large amounts of histamine from mast cells can exacerbate problems with histamine from foods. Too many mast cells or mast cells that are too easily activated can lead to mast cell activation syndrome (MCAS). Symptoms of MCAS include flushing, itchy skin, low blood pressure, constipation and/or diarrhea, abdominal pain, shortness of breath, and nasal congestion.[ref] The symptoms of MCAS are driven by excess histamine and overlap with histamine intolerance. Some researchers categorize histamine intolerance as a subset of mast cell activation syndrome (MCAS).
Related articles: Mast cell activation syndrome – Genetics, causes, solutions & MRGPRX2 receptor activation of mast cells
Histamine receptors: understand the different effects of histamine
You may wonder why one molecule can cause so many different actions in the body…
People ask, how can histamine cause headaches and heartburn and hives?
The function of histamine in a specific part of the body depends on the receptor it binds to.
Different histamine receptors are found in different parts of the body:[ref]
| Receptor | Location(s) | Main Effects |
|---|---|---|
| H1 | Smooth muscle, CNS, mast cells | Allergy symptoms, vasodilation, asthma |
| H2 | Stomach, blood vessels, heart | Stomach acid release, heart rhythm |
| H3 | Central/peripheral nervous system | Regulates brain histamine, neurotransmitters |
| H4 | Bone marrow, immune cells | Inflammatory response |
H1 receptors: Found in smooth muscle, endothelial cells (lining the blood vessels), the central nervous system, and mast cells. Activating the H1 receptors causes allergy-type symptoms such as itching, swelling, vasodilation, nose running, and skin reactions. H1 receptors are also important in asthma reactions.
H2 receptors: Acid is released when histamine activates the H2 receptors in the stomach. H2 receptors are also found in the intestinal tract and the walls of blood vessels. Mast cells also have H2 receptors, which, when activated, cause the release of more histamine. In the heart, H2 receptors are essential in controlling the rhythm.
H3 receptors: The central and peripheral nervous systems contain H3 receptors, which act as a feedback loop for histamine levels in the brain. Activating the H3 receptors impacts serotonin, norepinephrine, and acetylcholine release.[ref]
H4 receptors: These histamine receptors are at the core of the inflammatory response. H4 receptors are found in the bone marrow, basophils (a type of white blood cell), the thymus, small intestine, spleen, colon, and mast cells.[ref]
Histamine receptors in the gut:
The H1, H2, and H4 receptors all play a role in the way your intestines function, and this gives rise to the digestive problems seen in people with histamine intolerance.
- Histamine acts as a neurotransmitter involved in the contractions of the intestinal muscles.[ref]
- People with food allergies and IBS had significantly higher levels of H1 and H2 receptors in their intestines.[ref]
Genetics and Environment: Combined Effects on Histamine Intolerance
Genetic variants in the genes that encode the histamine-degrading enzymes are strongly linked to an increased relative risk of histamine intolerance. However, research shows that genetics alone is not usually the sole cause. Instead, environmental factors also play a role.
Genetics and Histamine Intolerance:
| Gene | Enzyme/Protein | Impact of Variants | Associated Conditions |
|---|---|---|---|
| AOC1 | DAO (diamine oxidase) | Reduced DAO activity, higher histamine | Migraines, food intolerance |
| HNMT | Histamine N-methyltransferase | Reduced CNS histamine breakdown | ADHD, migraines, eczema, asthma |
| HDC | Histidine decarboxylase | Altered histamine production | Tourette’s, heart conditions |
| MTHFR | Methylene tetrahydrofolate | Reduced methylation | Fewer methyl groups to break down histamine |
AOC1 gene: The AOC1 gene codes for the production of the DAO enzyme. DAO (diamine oxidase) is the enzyme produced in the intestines that breaks down histamine from foods. Genetic variants in AOC1 can increase or decrease the production of the enzyme. These variants are associated with increased risk of histamine intolerance, migraines, and ADHD. [ref][ref]
HNMT gene: Histamine N-methyltransferase (HMNT) is the enzyme that regulates histamine in the body via converting it from histamine into N-methylhistamine, which can then be eliminated from the body. HNMT is responsible for eliminating 80% of histamine in the body.[ref]
HDC gene: The HDC gene encodes an enzyme called histidine decarboxylase, which is used in the conversion of the amino acid histidine into histamine. The enzyme is found primarily in mast cells, neurons, basophils, and cells lining the stomach.[ref] Genetic variants in the HDC gene affect the susceptibility to Tourette’s syndrome and heart disease. [ref]
Methyl groups: The methylation cycle plays a role in breaking down monoamine neurotransmitters, including histamine. It is also important in creating the methyl groups needed for the HMNT enzyme to work. So, looking at your methylation cycle genes can also help with balancing out histamine intolerance.
MTHFR: The MTHFR gene codes for an enzyme that is a key player in the folate cycle. It is one source of methyl groups for the methylation cycle. Decreased enzyme activity of MTHFR, combined with a diet lacking in folate, may result in a reduced breakdown of histamine.
Histamine Receptor Genetic Variants: Histamine is a signal that is relayed by histamine receptors, and genetic variants in the receptors can increase the symptoms by enhancing the signal.
- HRH1 gene: Histamine receptor 1; this receptor is responsible for the allergy-type symptoms associated with histamine (nose running, eyes watering, itchy skin, airway reactivity). Variants in HRH1 affect the risk of allergies.
- HRH2 gene: Histamine receptor 2 is active in the production of stomach acid, the sinus node of the heart, and other places in the body. Variants in HRH2 affect the risk of gastritis, stomach pain, and stomach cancer.
- HRH3 gene: Histamine receptor 3 is important in regulating histamine levels in the brain. Genetic mutations in HRH3 impact the risk of chronic heart failure, and the demyelination of neurons in MS.[ref][ref]
- HRH4 gene: Histamine receptor 4 is found throughout the body, including in the brain. HRH4 receptors are also important in cancer progression.
Environmental Factors Affecting Histamine Intolerance:
For most, histamine intolerance is a combination of genetic susceptibility with environmental factors. By environment, I mean the things that make up your lifestyle – from your sleep to the gut microbiome to the chemicals you’re exposed to every day.
Histamine, Sleep, and Circadian Rhythm:
Histamine is a neurotransmitter in the brain. It is an alerting neurotransmitter, rising in the morning hours to wake us up. About 50% of the histamine in the brain is from mast cells.[ref]
Diphenhydramine (Benadryl) has the side effect of making people sleepy due to blocking the actions of histamine in the brain.
Altering histamine levels in the brain changes sleep:
- In mice, eliminating the histamine receptors in the brain alters sleep patterns. Without histamine, mice were slower to wake up. They also had fragmented sleep and decreased non-REM sleep.[ref]
- In another animal study, researchers decreased the number of mast cells in the brain, reducing histamine production. It did not affect the amount of time the mice slept overall, but it did affect their brain waves in sleep and their ability to bounce back after sleep deprivation.[ref]
In a recent study of people with suspected histamine intolerance, the researchers found that about 25% of the patients had a circadian change in histamine levels that differed from a control group. These patients had significantly reduced DAO enzyme levels and higher histamine levels during the day.[ref]
Your Gut Microbes Produce Histamine:
The gut microbiome significantly influences histamine levels. For some, histamine symptoms begin or worsen when trying to “fix the gut,” especially by consuming fermented foods, which are often high in histamine and may contain histamine-producing bacteria.
Some bacteria carry the HDC gene, which codes for an enzyme that converts L-histidine into histamine. These bacteria may also have transporters that import histidine and export histamine into the gut.[ref]
The gut microbiome in histamine intolerance:
When researchers looked at the gut microbiome, they found significant differences in the gut microbiomes of people with histamine intolerance compared to normal gut microbiomes. Histamine-secreting bacteria were abundant in people with histamine intolerance.[ref] Another study found that people with histamine intolerance found that they had a much lower abundance of Bifidobacteria in the gut and higher levels of Proteobacteria and Roseburia species. The phylum Proteobacteria includes Morganella morganii, which is strongly linked to histamine secretion.[ref]
Environmental chemicals that cause histamine release:
- Sodium fluoride primes mast cells to release histamine. [ref] The addition of sodium fluoride to drinking water is common in most US municipalities.
- PFOAs (Perfluorooctanoic acid) have been found to release histamine and cause mast cell degranulation. “… PFOA exacerbated allergic symptoms via hypothermia, and an increase of serum histamine, TNF-α, IgE, and IgG1 in the ovalbumin-induced systemic anaphylaxis. The present data indicate that PFOA aggravated FcɛRI-mediated mast cell degranulation and allergic symptoms.”[ref] You will find PFOAs in Teflon, stain-resistant carpeting, microwave popcorn bags, food wrappers, etc.
- Off-gassing from carpeting may cause mast cell release in the case of sick building syndrome.[ref]
- Sodium benzoate, a common preservative, causes histamine release in people with allergies and asthma.[ref]
- Aspirin and other salicylates can cause histamine problems for some people, possibly through basophil activation.[ref]
- Polysorbate 80, a common food additive, causes histamine release.[ref] Polysorbate 80 is in most brands of pickles — except organic pickles.
Medications that decrease DAO enzyme production
In addition to foods, drug interactions can cause a decrease in DAO enzyme production.
- Metformin has been shown to decrease the DAO enzyme.[ref]
- Vitamin B3 (nicotinamide or niacinamide) may increase histamine levels at doses of 100 mg or higher.[ref]
Making connections: Conditions that involve high histamine levels
When discussing histamine intolerance, the conversation often centers on foods and the symptoms affecting the gut or skin. However, elevated histamine levels can contribute to a variety of health conditions. By understanding how diet, histamine-degrading enzymes, the gut microbiome, and the body’s own histamine production are connected, you may be able to help other conditions related to high histamine levels.
Here are other conditions that involve high histamine levels:
- Asthma: Histamine release from mast cells is involved in the airway restriction in asthma.
- Brain fog: High histamine levels can be a cause of brain fog or cognitive issues
- Canker sores, oral lesions, or burning mouth.[ref]
- Periodic Limb Movement Disorder: Animal studies show that H3 receptor activation causes limb movements during sleep
- Autoinflammatory conditions, such as familial Mediterranean fever: People with FMF have higher-than-normal histamine levels during a flare-up.
- Mast Cell Activation Syndrome: MCAS is like histamine intolerance on steroids.
- Ehlers-Danlos Hypermobility Syndrome: Mast cells and high histamine in the joints are a possible underlying cause of hypermobility syndrome.
- Migraines: Genetic variants in the AOC1 (DAO) gene increase the risk of migraines.
- ADHD: Histamine-related variants increase the risk of ADHD
- Early morning waking / insomnia: One underlying cause of early morning insomnia is that high histamine levels cause too much alertness.
- Heart rhythm issues, including atrial fibrillation: The H2 receptor and histamine play an integral role in heart rhythm.
- Fibromyalgia: Mast cell activation and histamine release could be adding to fibromyalgia symptoms.
- COPD: Histamine levels and mast cell activation cause bronchial constriction in COPD.[ref][ref]
- Tinnitus and Meniere’s: A genetic variant in the H4 receptor increases the risk of Meniere’s and ringing in the ears.
- Pollen allergies and food allergies: Mast cells release histamine when triggered by an allergen.
How can you use this information?
Histamine intolerance – or rather, overall high histamine levels – could be an additive factor for the above conditions.
If you have a condition that is caused in part by high histamine levels, understanding the dietary and gut microbiome connections to histamine can be a game-changer for some people. For example, if you have brain fog or ringing in your ears, trialing a low-histamine diet for a week or two may help you determine if high-histamine foods are playing a role in your symptoms. Similarly, if you have fibromyalgia, looking at the role of mast cells and histamine may lead to supplements, such as quercetin or DAO, along with a low-histamine diet as a solution.
Genotype Report: Histamine Intolerance
Access this content:
An active subscription is required to access this content.
Lifehacks for histamine intolerance:
Below are the research-backed solutions for histamine intolerance. You may need to try several different ‘lifehacks’ to see which works best for you.
Low-histamine diet:
A low-histamine diet restricts foods with high levels of histamine or that cause the body to release histamine. To experiment with a low-histamine diet, eliminate all of the higher-histamine foods for a period of time to see how your body responds.
In general, foods that are fermented or aged are higher in histamine. High histamine foods include processed meats, cheeses (except farmer cheese), fish and seafood that isn’t completely fresh, spinach, chocolate, tomatoes, strawberries, wine, sake, and more.
High histamine foods list:
If you are considering a low histamine diet, I find this histamine food list to be the most thorough:
Complete list of foods that are high in histamine (pdf).
Here are the highlights of what to avoid on a low-histamine diet:
| Raw egg whites | Buckwheat, malted barley | Chickpeas |
| Blue Cheese | Walnuts, pecans | Lentils |
| Hard cheeses (mature) | Avocados | Spinach |
| Dried meat (jerky) | Eggplant | Soybeans, edamame |
| Cured ham | Hot peppers | Tomatoes |
| Most organ meats | Anything pickled | Bananas (very ripe) |
| Processed meats | Citrus fruits | Chocolate |
| Smoked meat, BBQ | Guava | Kiwi |
| Anchovies | Pineapple | Strawberries |
| Fish (not very fresh) | Nori, algae | Cumin |
| Meat (not fresh, leftovers) | Mustard seeds | Soy sauce |
| Seafood (except ‘frozen at sea’) | Vinegar |
You may find that some of these foods don’t cause histamine reactions, so it is a matter of trial and error to find out what works best for you. For example, soy sauce causes histamine reactions in many people, but it may depend on which species of bacteria is used in the fermentation process. Certain bacteria can reduce the histamine content of soy sauce.[ref]
Note that alcohol can cause mast cells to release histamine. For some, this may cause flushing when drinking. But for people with histamine intolerance, even alcoholic drinks that are lower in histamine may still cause a reaction.[ref]
Related article: Alcohol, mast cells, and histamine
What does a low-histamine diet do?
- Decreasing the amount of histamine you take into your body will lower the overall amount of histamine circulating in your body.
- Research studies show that a low histamine diet helps with chronic urticaria (itchiness, hives), migraines, stomach problems, and asthma.[ref][ref]
Should you maintain a low histamine diet long-term?
Trying a low-histamine diet for a period of time can give you a lot of insight into how histamine affects your body, but it may not be a diet you want to continue long-term. A low-histamine diet restricts many healthy foods that you may enjoy, such as spinach, strawberries, and avocados.
Use a low-histamine diet as a tool to learn which histamine-containing foods bother you the most. It can also be a short-term way of getting histamine responses under control.
Low FODMAPs diet: histamine and gut problems
Interestingly, a randomized controlled study for people diagnosed with IBS found that a low FODMAPs diet reduced symptoms and reduced histamine levels.
It could mean that a FODMAPs diet works because IBS is related to histamine intolerance – or – it could mean that the people diagnosed with IBS were really dealing with gut-related histamine symptoms.[ref] Additionally, the low FODMAPs diet may help to decrease intestinal barrier permeability.
A low FODMAPs diet cuts out a lot of high-histamine foods, so it could reduce histamine levels by eating fewer foods high in histamine. On the other hand, a low FODMAPs diet impacts the gut microbiome and histamine-producing bacteria. Animal studies also link IBS to mast cell activation in the colon, so changing the gut microbiome with a FODMAPs diet may also impact colonic mast cells.[ref]
Learn more about what is included in a low FODMAPs diet: Starting a Low FODMAPs diet
Related article: Genetic reasons the FODMAPs diet doesn’t work
Supplements for Histamine Intolerance:
When looking at natural supplement for histamine intolerance,
Access this content:
An active subscription is required to access this content.
Related Articles and Topics:
References:
Gilligan, Gerardo, and María Fernanda Galindez-Costa. “Histamine Intolerance: A Pioneering Report on the Oral Manifestations of a Complex Systemic Disorder.” Oral Diseases, vol. 31, no. 10, Oct. 2025, pp. 2857–64. PubMed, https://doi.org/10.1111/odi.15363.
Agúndez, J. A. G., Ayuso, P., Cornejo-García, J. A., Blanca, M., Torres, M. J., Doña, I., Salas, M., Blanca-López, N., Canto, G., Rondon, C., Campo, P., Laguna, J. J., Fernández, J., Martínez, C., & García-Martín, E. (2012). The diamine oxidase gene is associated with hypersensitivity response to non-steroidal anti-inflammatory drugs. PLoS ONE, 7(11), e47571. https://doi.org/10.1371/journal.pone.0047571
Andersen, L. P. H., Werner, M. U., Rosenkilde, M. M., Harpsøe, N. G., Fuglsang, H., Rosenberg, J., & Gögenur, I. (2016). Pharmacokinetics of oral and intravenous melatonin in healthy volunteers. BMC Pharmacology & Toxicology, 17, 8. https://doi.org/10.1186/s40360-016-0052-2
Association of diamine oxidase and histamine N-methyltransferase polymorphisms with presence of migraine in a group of Mexican mothers of children with allergies. (2017). Neurología (English Edition), 32(8), 500–507. https://doi.org/10.1016/j.nrleng.2016.02.012
Ayuso, P., García-Martín, E., Martínez, C., & Agúndez, J. A. G. (2007). Genetic variability of human diamine oxidase: Occurrence of three nonsynonymous polymorphisms and study of their effect on serum enzyme activity. Pharmacogenetics and Genomics, 17(9), 687–693. https://doi.org/10.1097/FPC.0b013e328012b8e4
Che, D. N., Cho, B. O., Shin, J. Y., Kang, H. J., Kim, Y.-S., & Jang, S. I. (2018). Fisetin inhibits IL-31 production in stimulated human mast cells: Possibilities of fisetin being exploited to treat histamine-independent pruritus. Life Sciences, 201, 121–129. https://doi.org/10.1016/j.lfs.2018.03.056
Chikahisa, S., Kodama, T., Soya, A., Sagawa, Y., Ishimaru, Y., Séi, H., & Nishino, S. (2013a). Histamine from brain resident mast cells promotes wakefulness and modulates behavioral states. PLoS ONE, 8(10), e78434. https://doi.org/10.1371/journal.pone.0078434
Chikahisa, S., Kodama, T., Soya, A., Sagawa, Y., Ishimaru, Y., Séi, H., & Nishino, S. (2013b). Histamine from brain resident mast cells promotes wakefulness and modulates behavioral states. PLoS ONE, 8(10), e78434. https://doi.org/10.1371/journal.pone.0078434
García-Martín, E., Ayuso, P., Martínez, C., Blanca, M., & Agúndez, J. A. G. (2009). Histamine pharmacogenomics. Pharmacogenomics, 10(5), 867–883. https://doi.org/10.2217/pgs.09.26
García-Martín, E., Martínez, C., Serrador, M., Alonso-Navarro, H., Ayuso, P., Navacerrada, F., Agúndez, J. A. G., & Jiménez-Jiménez, F. J. (2015). Diamine oxidase rs10156191 and rs2052129 variants are associated with the risk for migraine. Headache, 55(2), 276–286. https://doi.org/10.1111/head.12493
Hon, Y. Y., Jusko, W. J., Zhou, H.-H., Chen, G.-L., Guo, D., Zhou, G., Spratlin, V. E., & Jann, M. W. (2006). Endogenous histamine and cortisol levels in subjects with different histamine n-methyltransferase c314t genotypes. Molecular Diagnosis & Therapy, 10(2), 109–114.
Maintz, L., Yu, C.-F., Rodríguez, E., Baurecht, H., Bieber, T., Illig, T., Weidinger, S., & Novak, N. (2011). Association of single nucleotide polymorphisms in the diamine oxidase gene with diamine oxidase serum activities. Allergy, 66(7), 893–902. https://doi.org/10.1111/j.1398-9995.2011.02548.x
McIntosh, K., Reed, D. E., Schneider, T., Dang, F., Keshteli, A. H., De Palma, G., Madsen, K., Bercik, P., & Vanner, S. (2017). FODMAPs alter symptoms and the metabolome of patients with IBS: A randomised controlled trial. Gut, 66(7), 1241–1251. https://doi.org/10.1136/gutjnl-2015-311339
Piliponsky, A. M., Acharya, M., & Shubin, N. J. (2019). Mast cells in viral, bacterial, and fungal infection immunity. International Journal of Molecular Sciences, 20(12), E2851. https://doi.org/10.3390/ijms20122851
Pinzer, T. C., Tietz, E., Waldmann, E., Schink, M., Neurath, M. F., & Zopf, Y. (2018). Circadian profiling reveals higher histamine plasma levels and lower diamine oxidase serum activities in 24% of patients with suspected histamine intolerance compared to food allergy and controls. Allergy, 73(4), 949–957. https://doi.org/10.1111/all.13361
Sander, L. E., Lorentz, A., Sellge, G., Coëffier, M., Neipp, M., Veres, T., Frieling, T., Meier, P. N., Manns, M. P., & Bischoff, S. C. (2006). Selective expression of histamine receptors H1R, H2R, and H4R, but not H3R, in the human intestinal tract. Gut, 55(4), 498–504. https://doi.org/10.1136/gut.2004.061762
Schnedl, W. J., Lackner, S., Enko, D., Schenk, M., Holasek, S. J., & Mangge, H. (2019). Evaluation of symptoms and symptom combinations in histamine intolerance. Intestinal Research, 17(3), 427–433. https://doi.org/10.5217/ir.2018.00152
Simon, T., Semsei, A. F., Ungvári, I., Hadadi, E., Virág, V., Nagy, A., Vangor, M. S., László, V., Szalai, C., & Falus, A. (2012). Asthma endophenotypes and polymorphisms in the histamine receptor HRH4 gene. International Archives of Allergy and Immunology, 159(2), 109–120. https://doi.org/10.1159/000335919
Stevenson, J., Sonuga-Barke, E., McCann, D., Grimshaw, K., Parker, K. M., Rose-Zerilli, M. J., Holloway, J. W., & Warner, J. O. (2010). The role of histamine degradation gene polymorphisms in moderating the effects of food additives on children’s adhd symptoms. American Journal of Psychiatry, 167(9), 1108–1115. https://doi.org/10.1176/appi.ajp.2010.09101529
Thangam, E. B., Jemima, E. A., Singh, H., Baig, M. S., Khan, M., Mathias, C. B., Church, M. K., & Saluja, R. (2018). The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: The hunt for new therapeutic targets. Frontiers in Immunology, 0. https://doi.org/10.3389/fimmu.2018.01873
Threlfell, S., Cragg, S. J., Kalló, I., Turi, G. F., Coen, C. W., & Greenfield, S. A. (2004). Histamine H3 receptors inhibit serotonin release in substantia nigra pars reticulata. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 24(40), 8704–8710. https://doi.org/10.1523/JNEUROSCI.2690-04.2004
Weng, Z., Zhang, B., Asadi, S., Sismanopoulos, N., Butcher, A., Fu, X., Katsarou-Katsari, A., Antoniou, C., & Theoharides, T. C. (2012). Quercetin is more effective than cromolyn in blocking human mast cell cytokine release and inhibits contact dermatitis and photosensitivity in humans. PloS One, 7(3), e33805. https://doi.org/10.1371/journal.pone.0033805
Yoshikawa, T., Nakamura, T., & Yanai, K. (2019). Histamine n-methyltransferase in the brain. International Journal of Molecular Sciences, 20(3), 737. https://doi.org/10.3390/ijms20030737
Yu, X., Ma, Y., Harding, E. C., Yustos, R., Vyssotski, A. L., Franks, N. P., & Wisden, W. (2019). Genetic lesioning of histamine neurons increases sleep–wake fragmentation and reveals their contribution to modafinil-induced wakefulness. Sleep, 42(5), zsz031. https://doi.org/10.1093/sleep/zsz031
Originally published April, 2015.